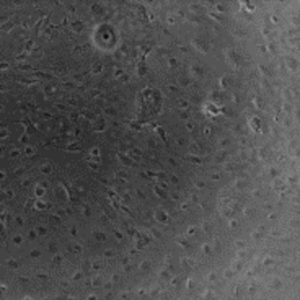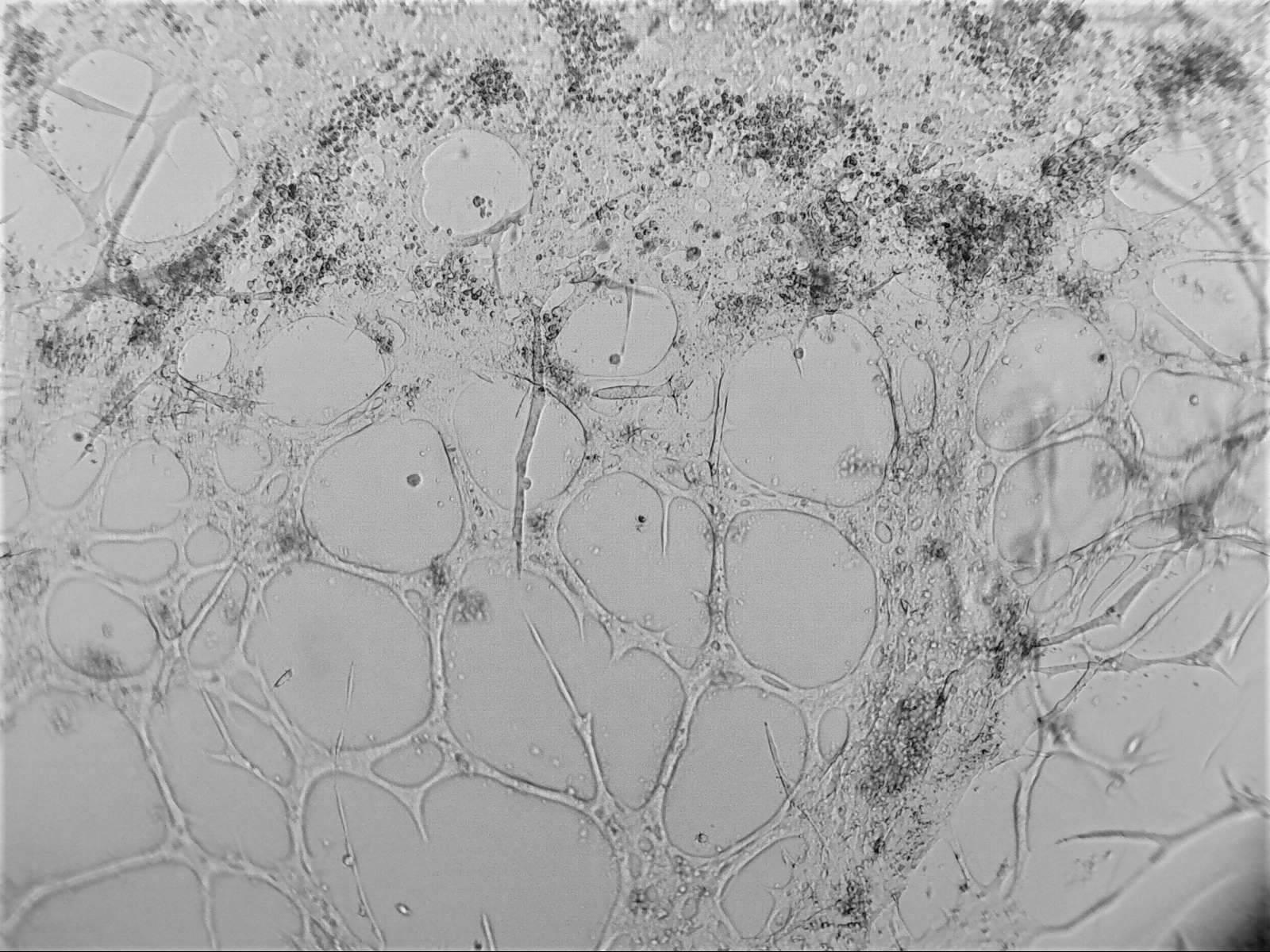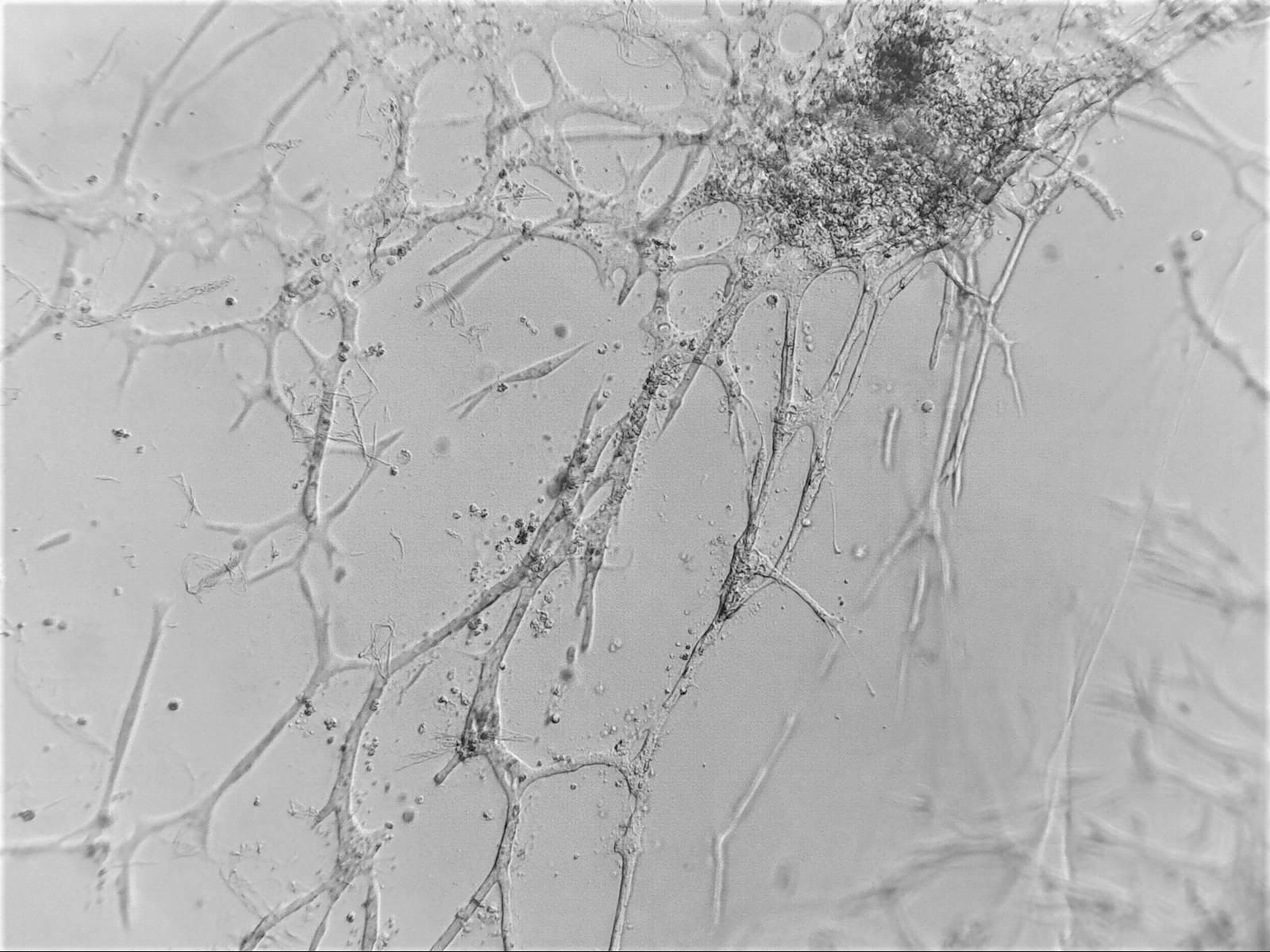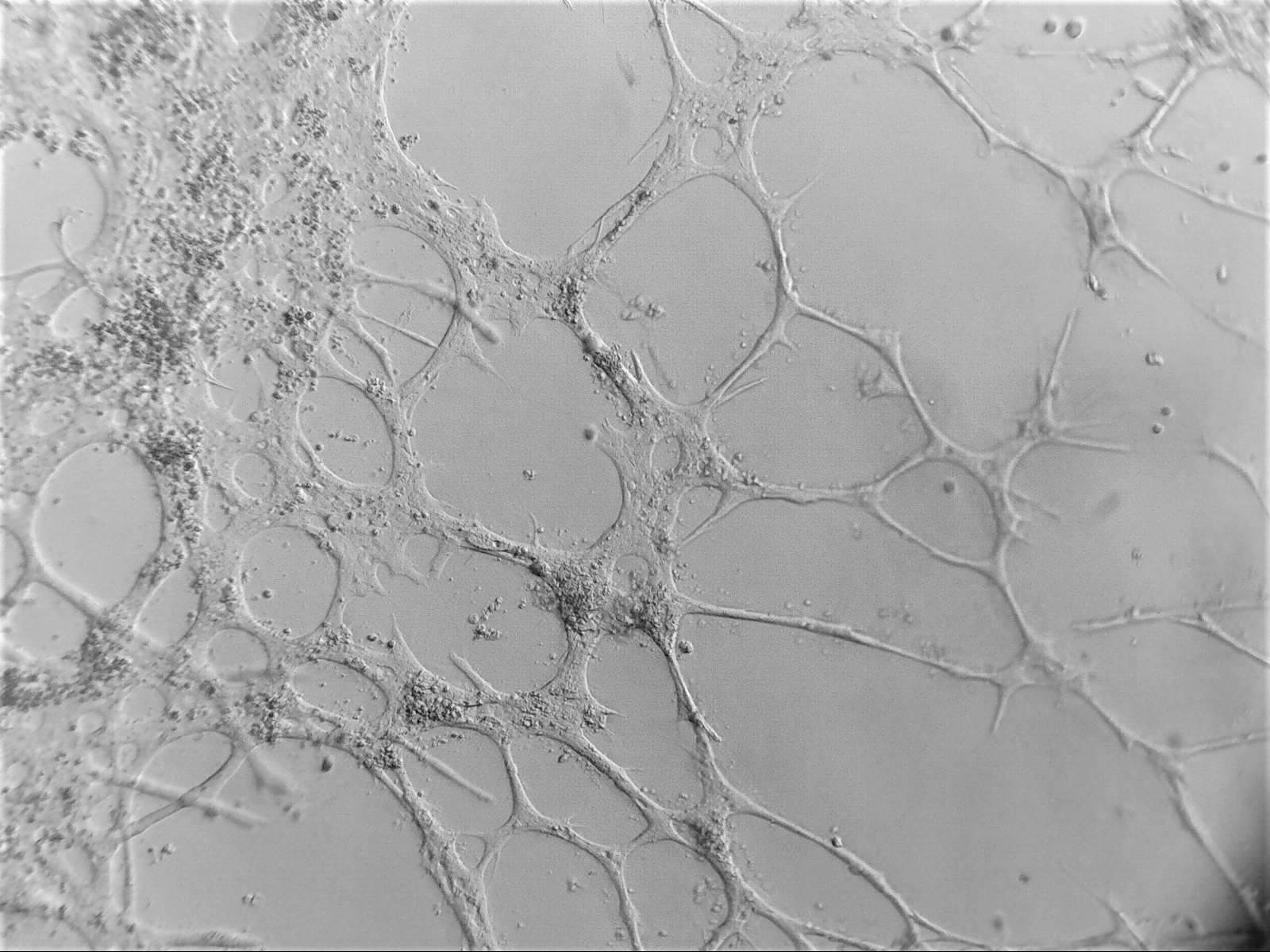
Cell lines in 3D
We have worked with a wide range of cell lines. Below, you’ll find our recommended cell lines and sample images.
| Pancreatic cancer | Mouse: PAN02 Human: PANC1, AsPC1, CFPAC1, MiaPaca2 | |
| Lung adenocarcinoma | Human: HCC827, H441, H1650, H1975, HCC 4006, H1993, A549 | |
| Ovarian cancer | Human: OVCAR3, COV318, OV-90, UWB1.289 + BRCA1, SKOV3 | |
| Endometrial carcinoma | Human: RL95-2 | |
| Endometrial cells | Human stromal: HESC, SHT290, Human endometrial: 12Z | |
| Cervical cancer | Human: HeLa | |
| Breast cancer | Human: MCF7, MCF10, SKBR3, MDA-MB 231, Mouse: 4T1 | |
| Colorectal carcinoma | Human: RKO, LoVo, LS180 | |
| Hepatocellular carcinoma | Human: Hep3B | |
| Prostate cancer | Human: PC3 | |
| Kidney cancer | Human: RCC | |
| Bone osteosarcoma | Human: U-2 OS | |
| Glioblastoma | Human: A172, T98G, U87, LN229, Mouse: GL261 | |
| Melanoma | Mouse: B16-F10 | |
| Stem cell | iPSC, hMSC, BM-MSC, AD-MSC, skeletal muscle-derived MSC, MSC isolated from horses | |
| Fibroblast | Mouse: L929 | |
| Monocyte | Human: THP1 | |
| Co-culture | CHO cells with Jurkat T, RL95-2 with PBMC | |
| Primary cells | HUVEC, colon cancer PDX, breast cancer PDX, HAEC | |
| Other | Chinese hamster ovary (CHO, Human Embryonic Kidney (HEK 293), Caco-2 intestinal drug transporter model (inserts with LifeGel), T78-1, DIPG (Diffuse Intrinsic Pontine Glioma) | |
Can't find your cell line on the list? Contact us – we’ll help you optimize your 3D cell culture or handle it for you.
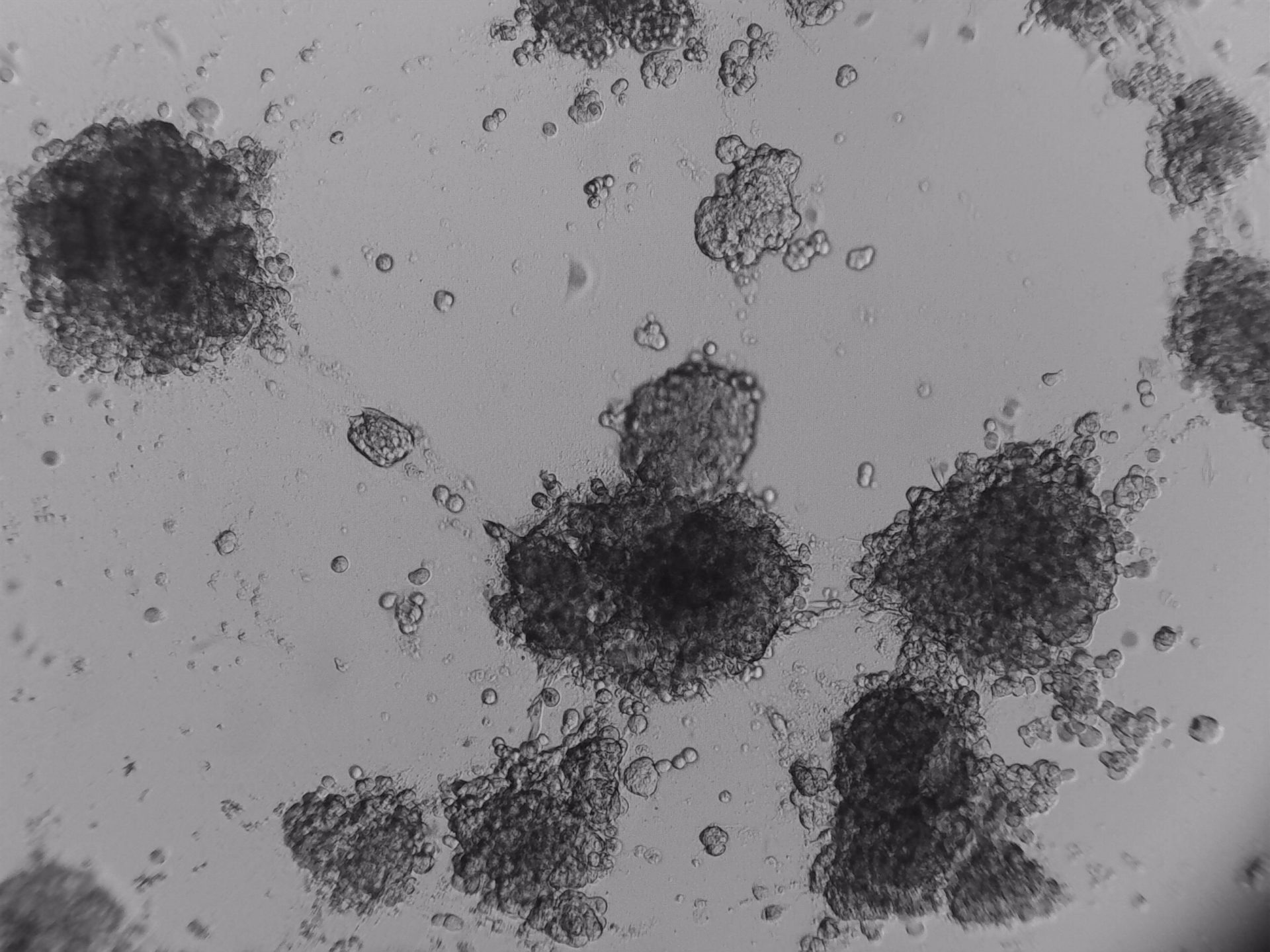
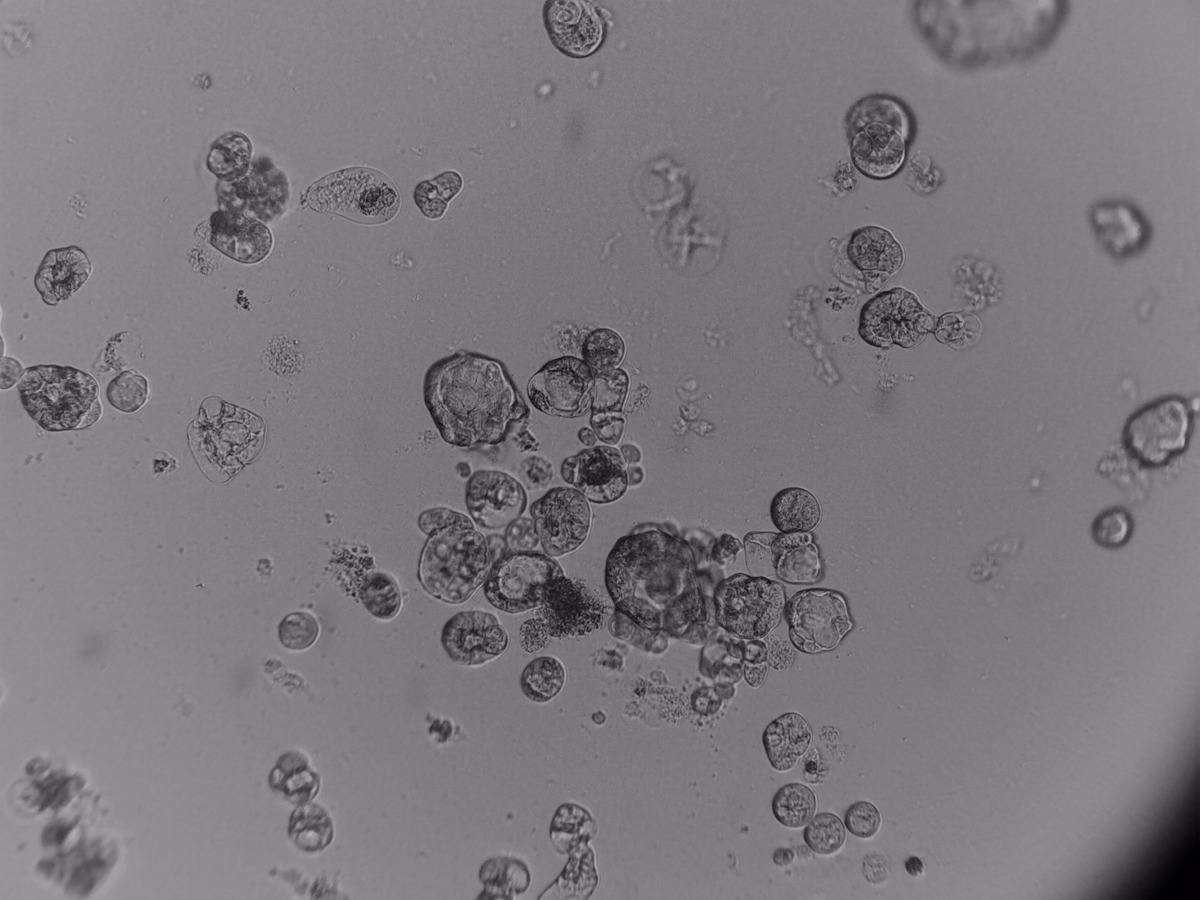
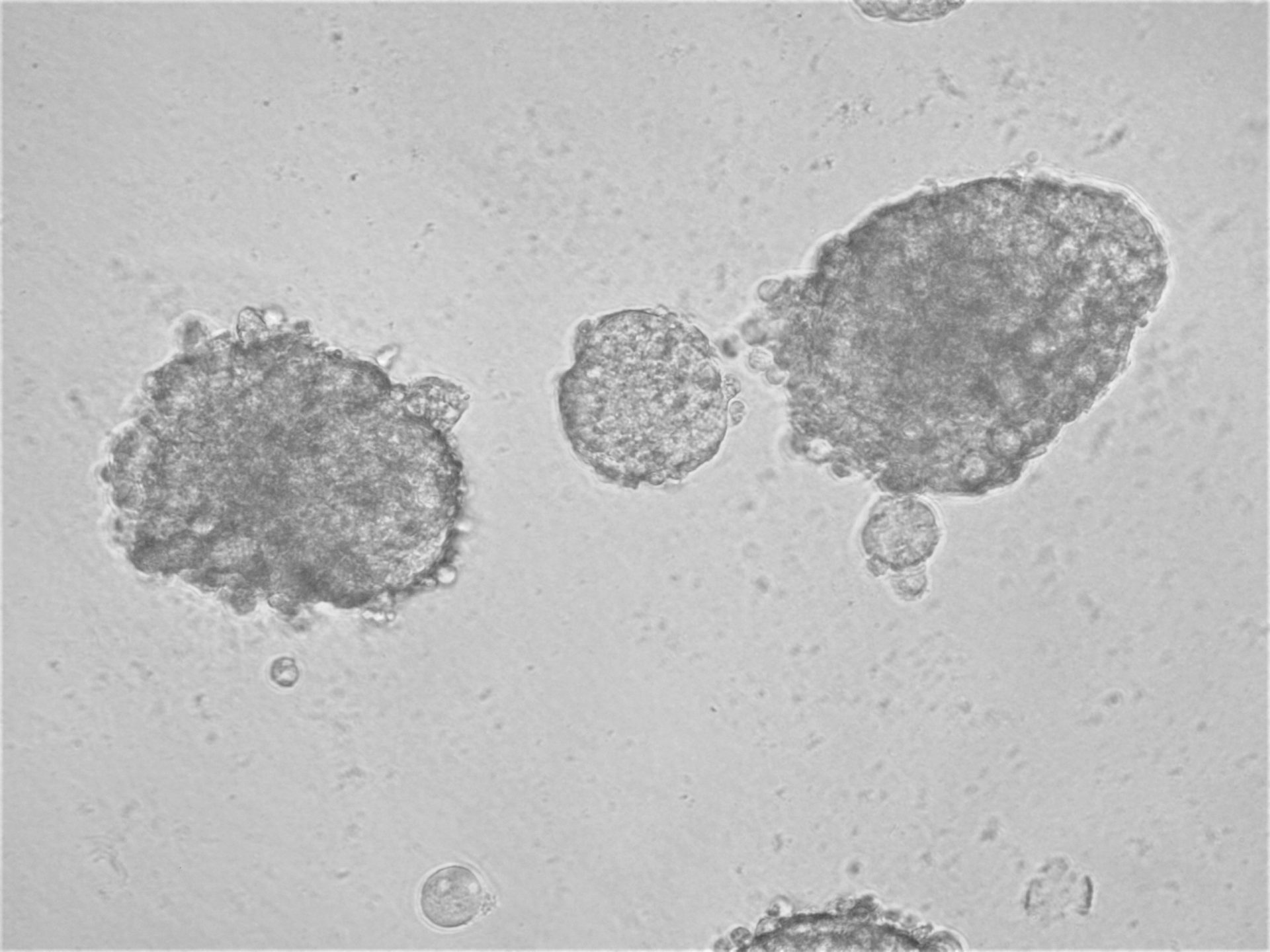
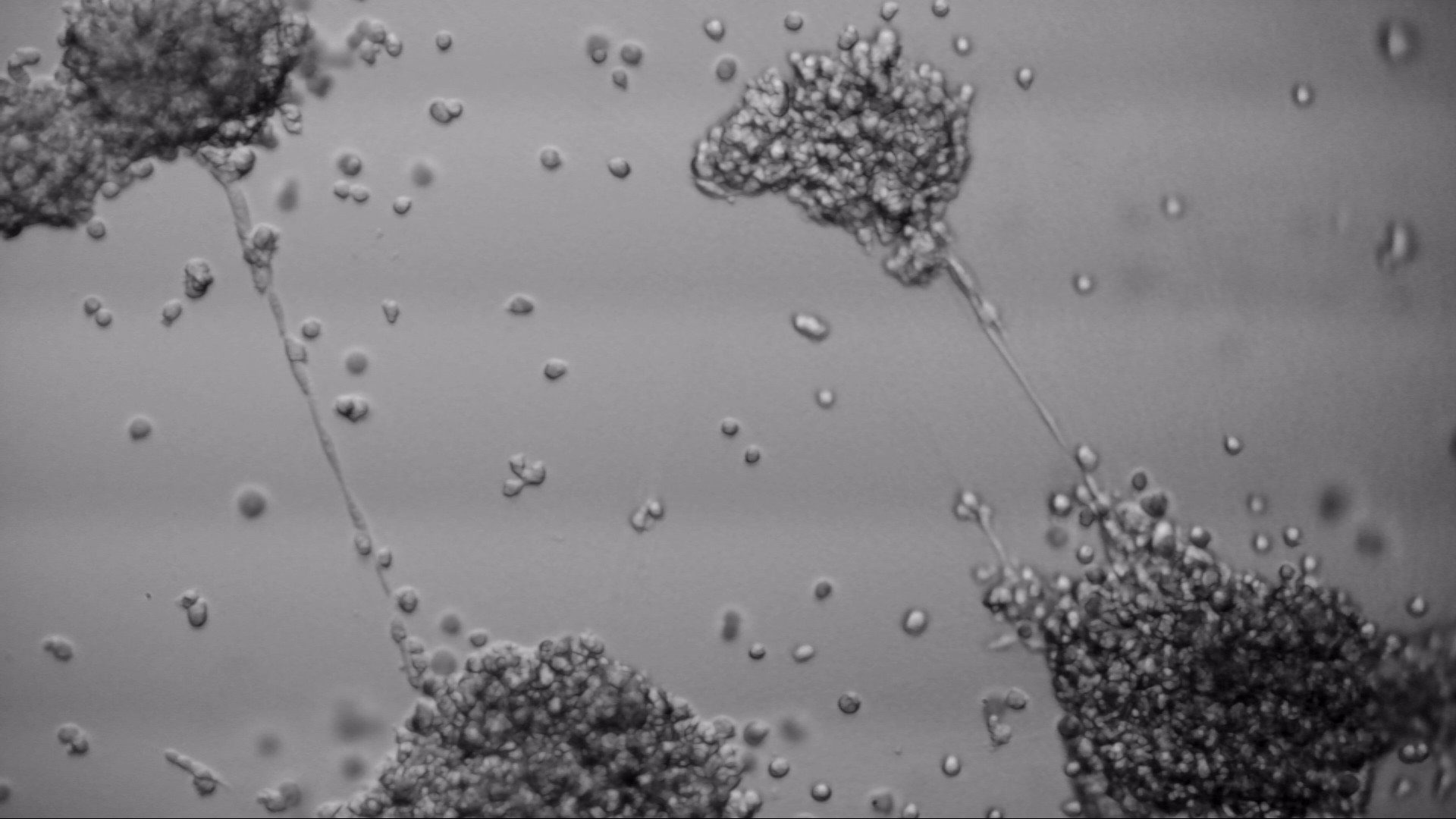
Pancreatic cell lines
On the photos below there is results of Panc-1 triple staining designed for staining different cellular organels – DiO (green) for cell membranes, MitoLite (red) for mitochondria and Hoechst (blue) for nuclei.
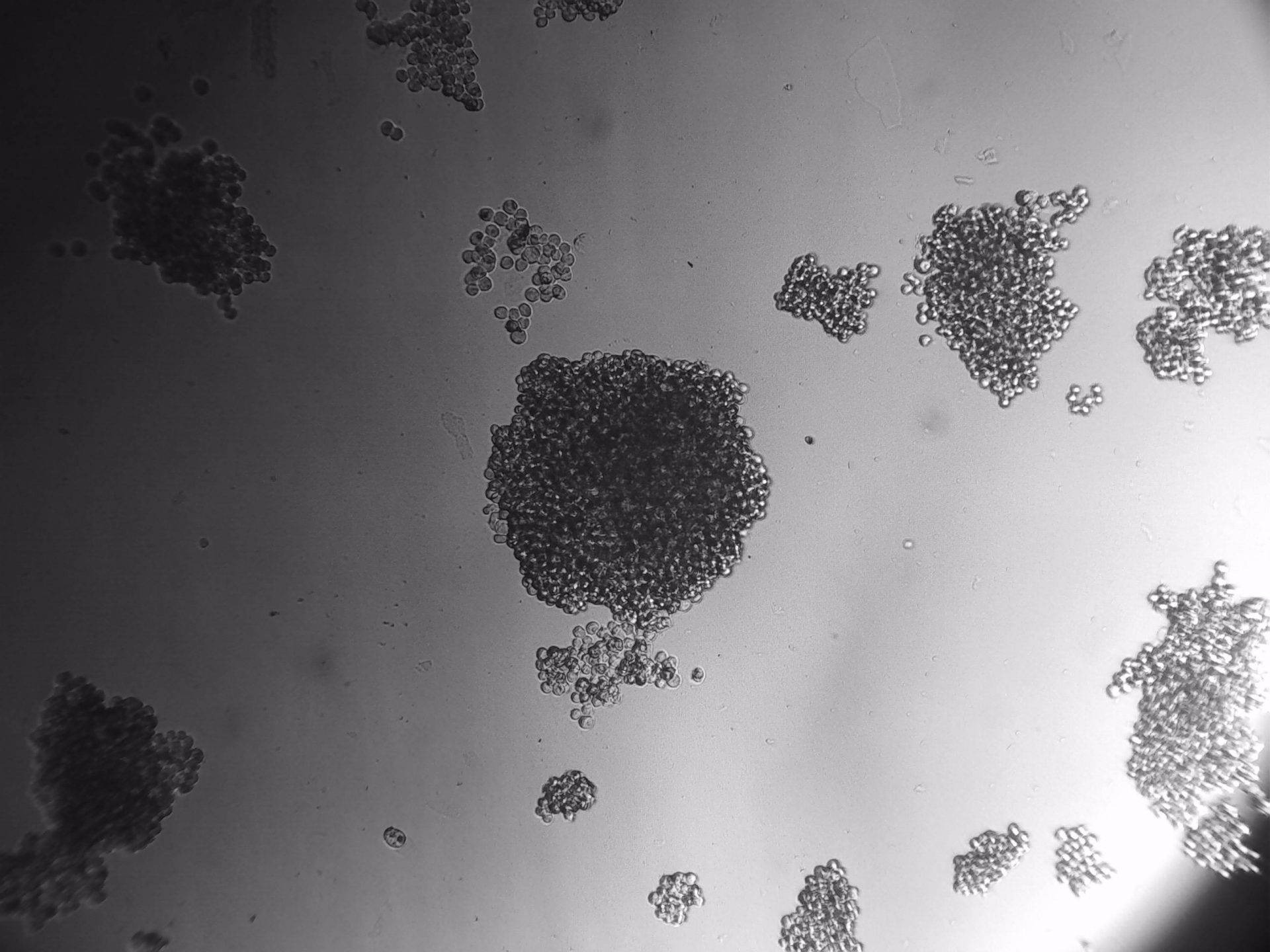
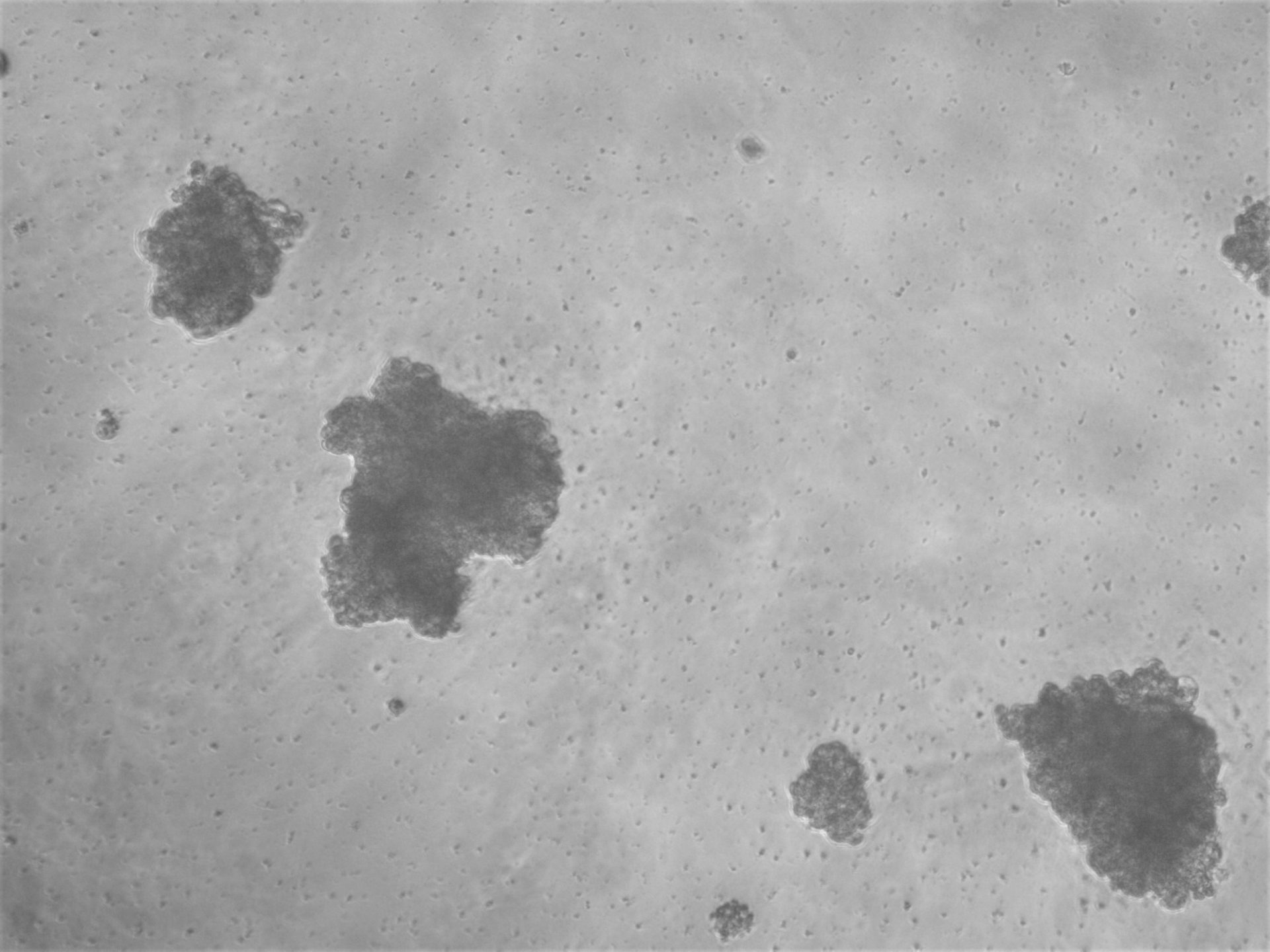
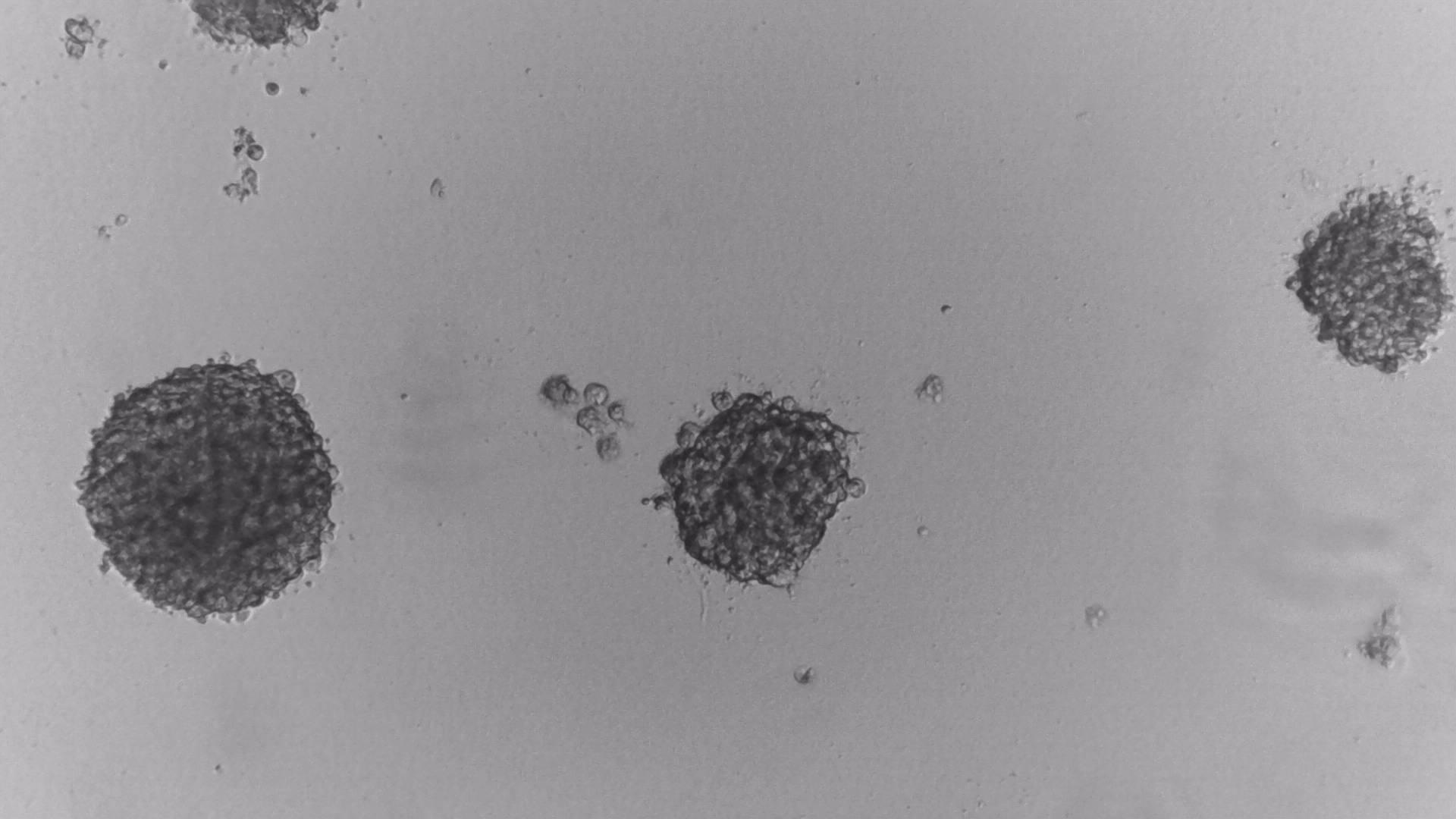
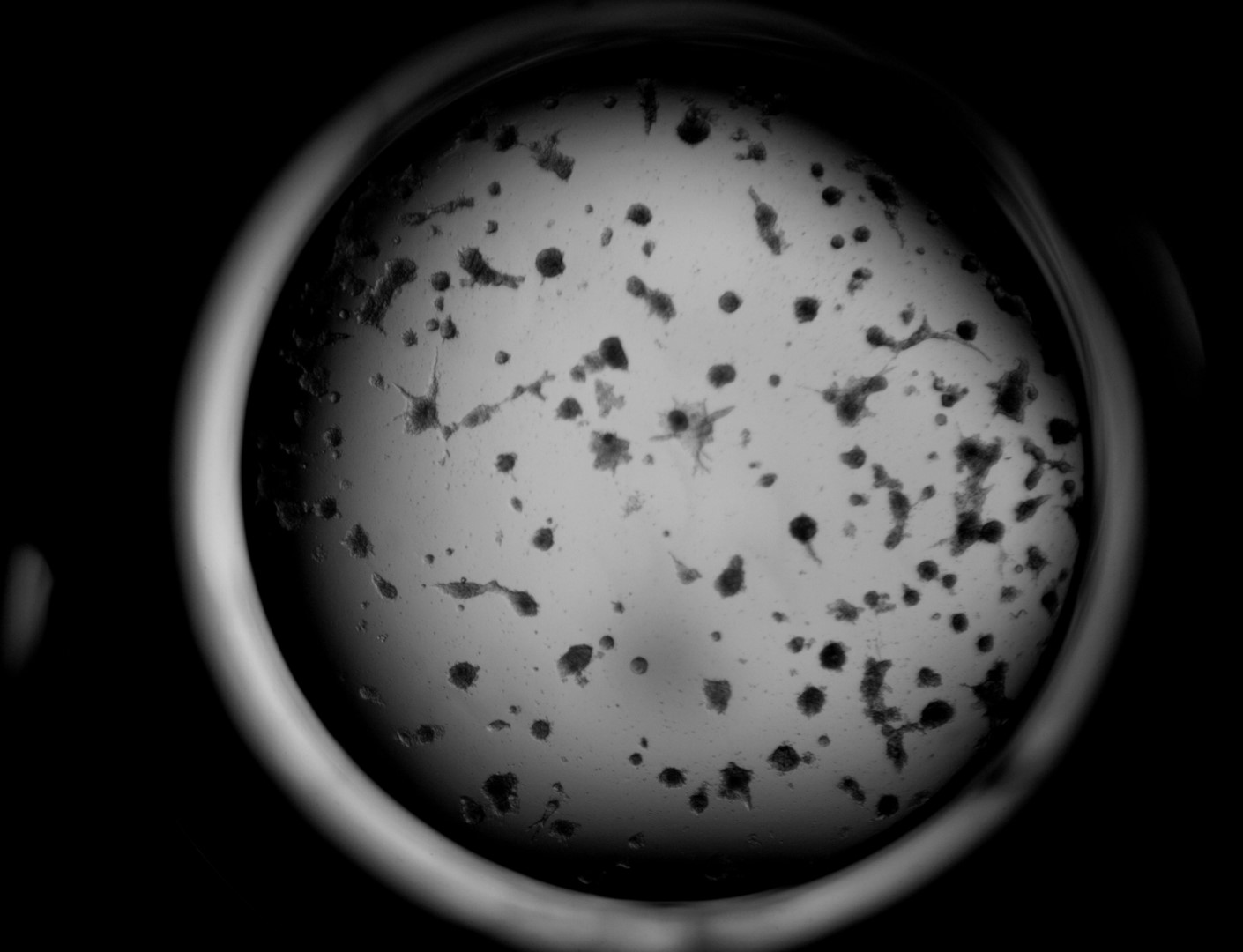
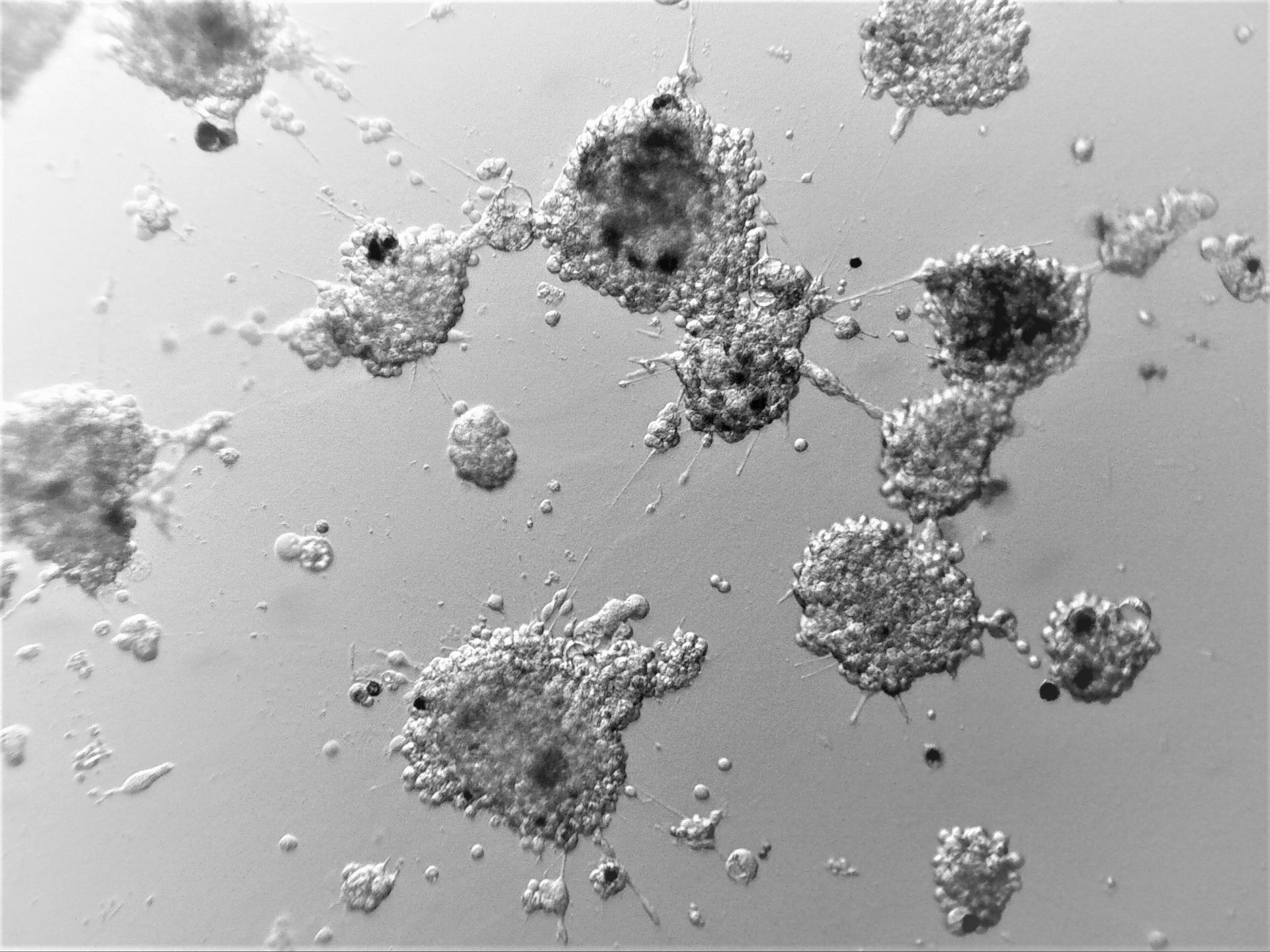
MiaPaca-2
Cells from photo on the left and in the middle have been cultured on LifeGel 48-well plates for 10-14 days, 2500 cells per well have been seeded. On the photo on the right, cells were cultured for 10 days on 96-well plate, they have been seeded 1000 cells per well.
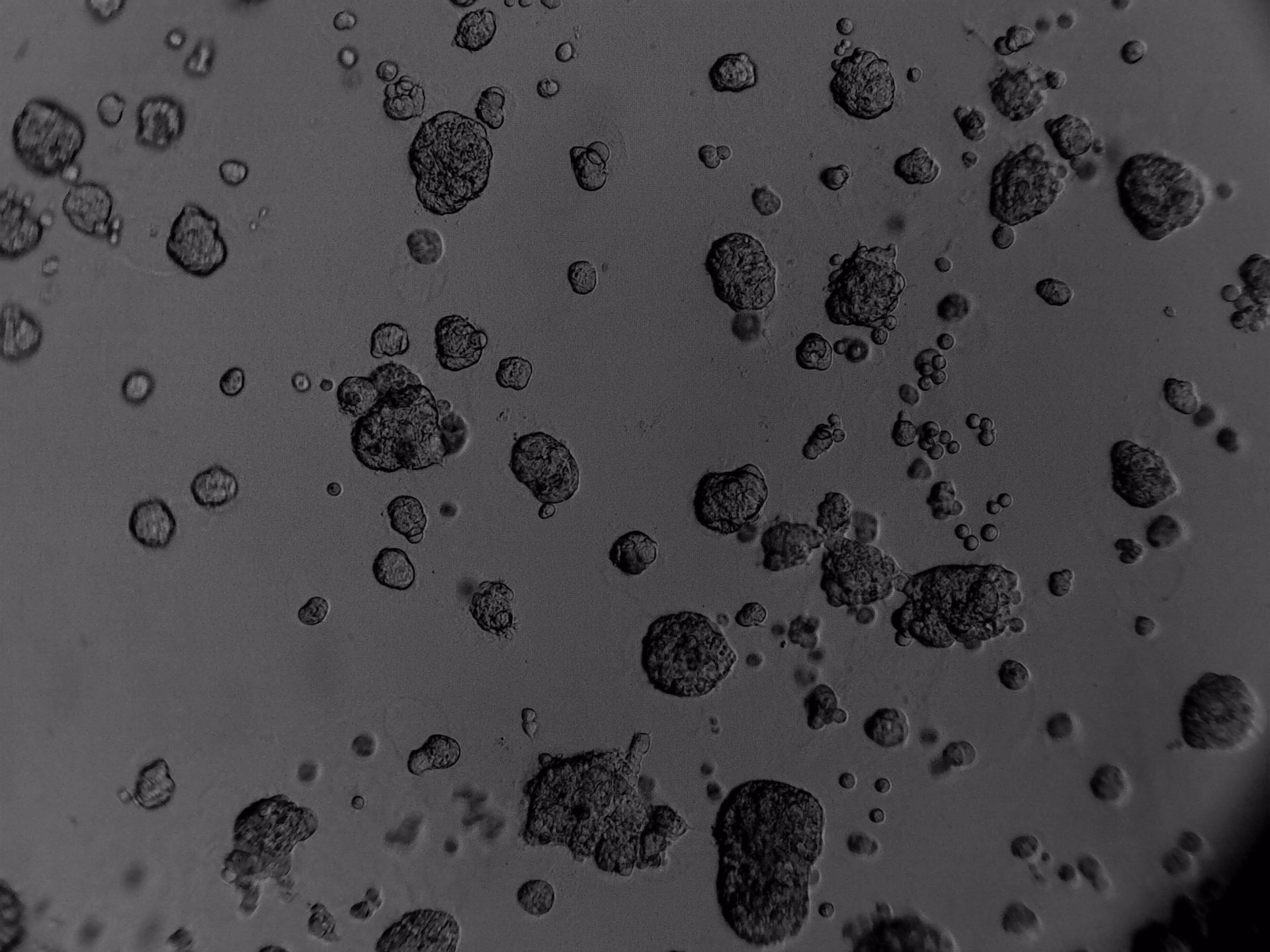
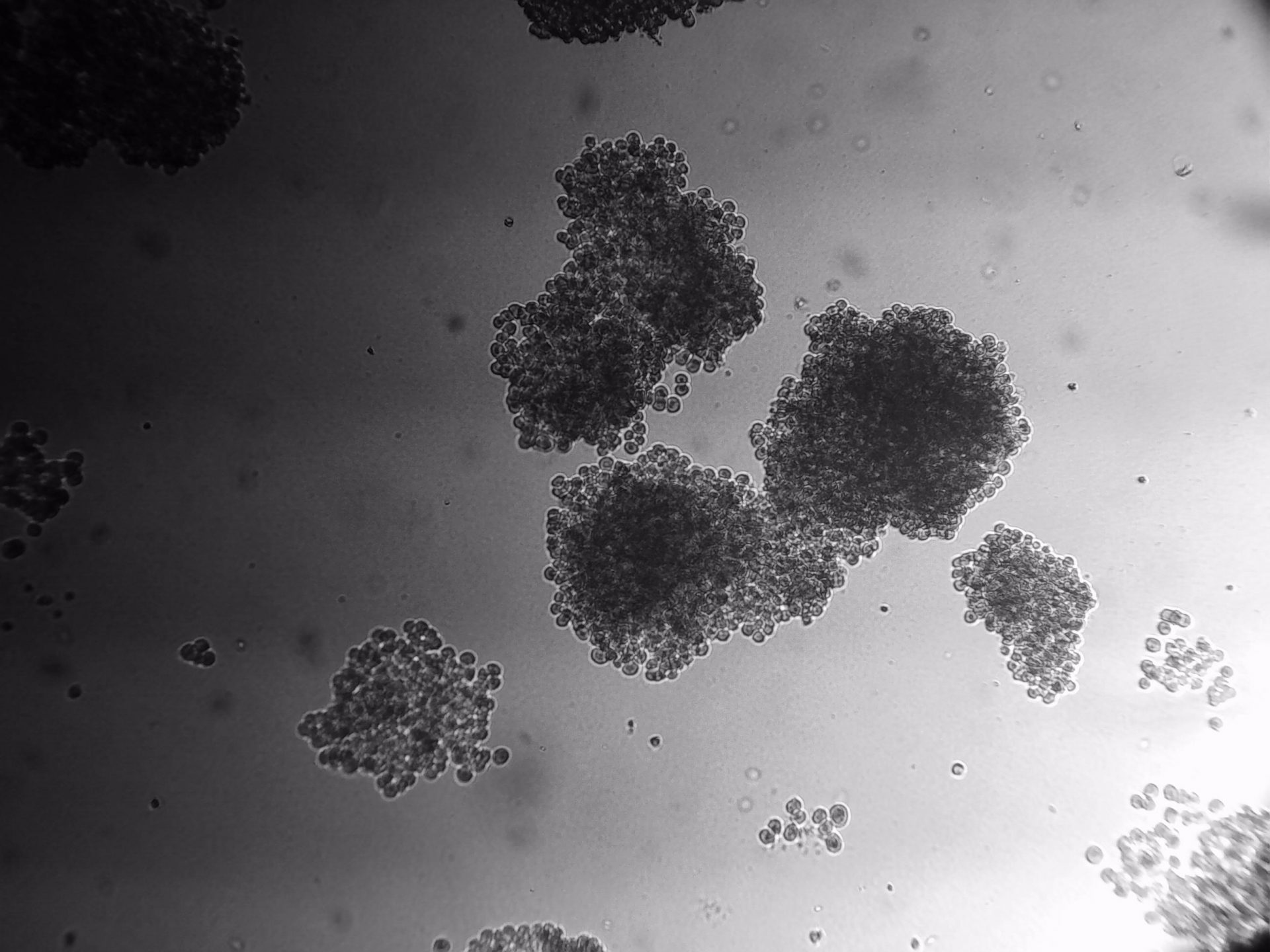
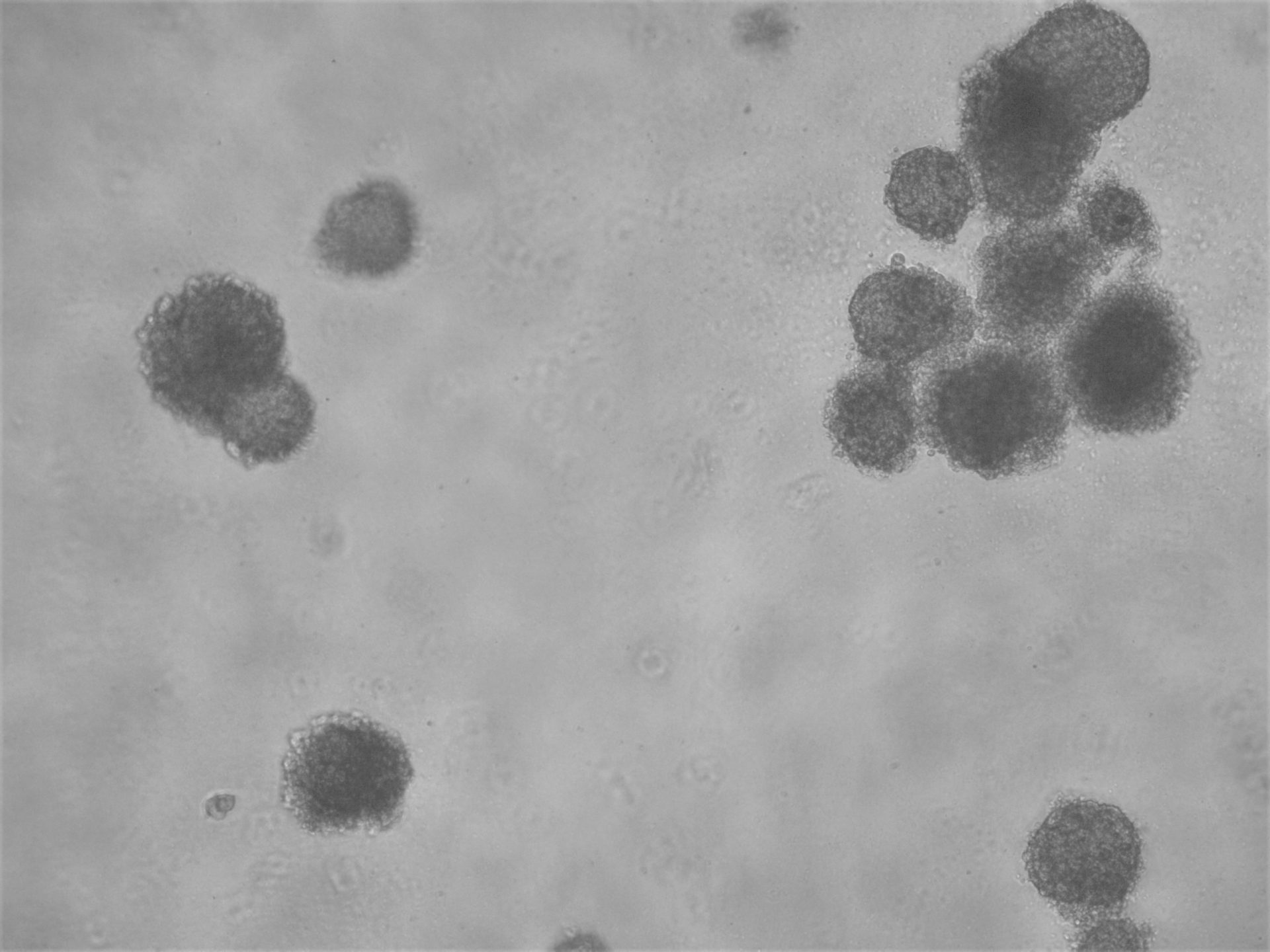
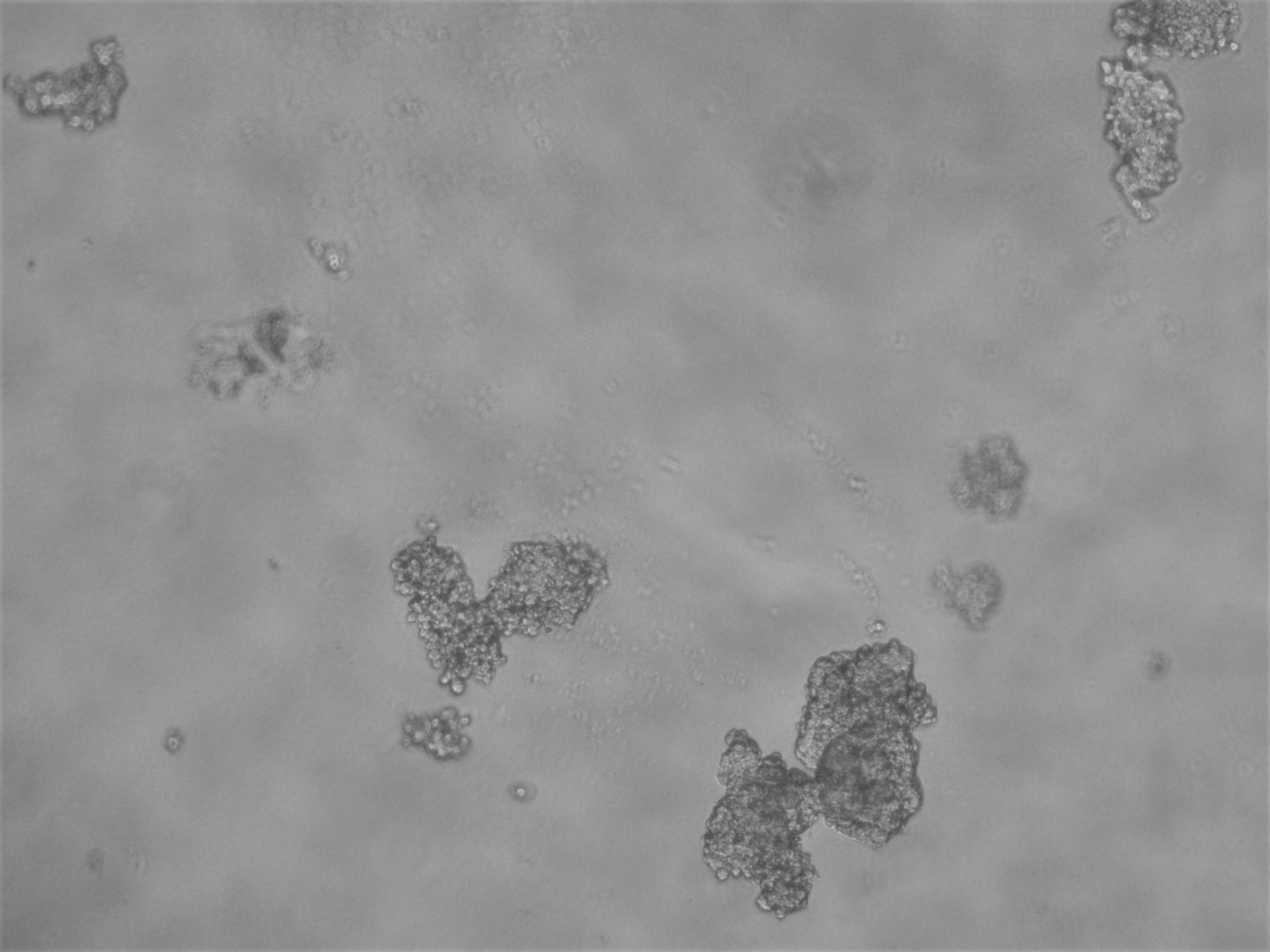
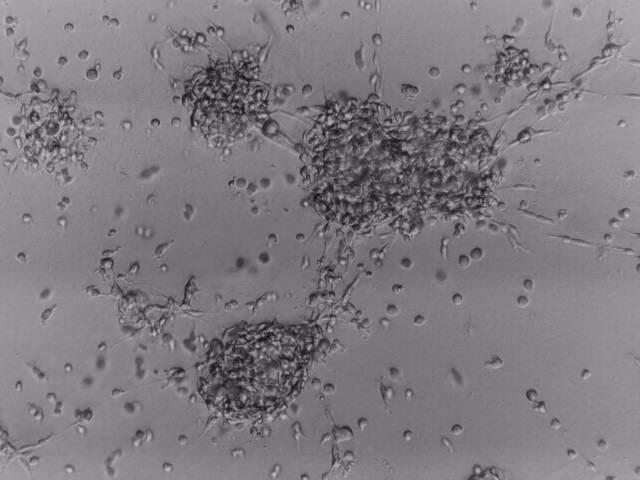
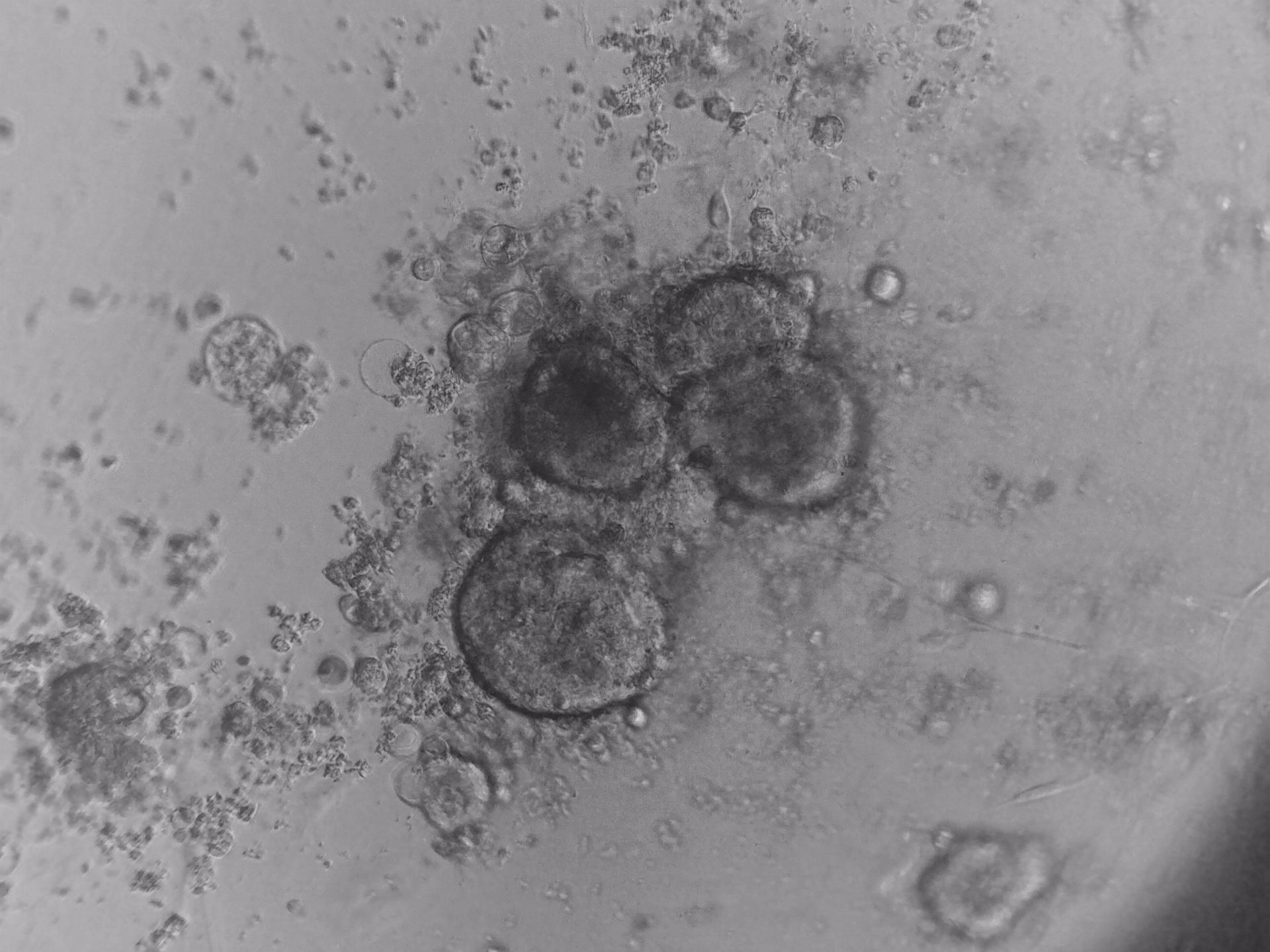
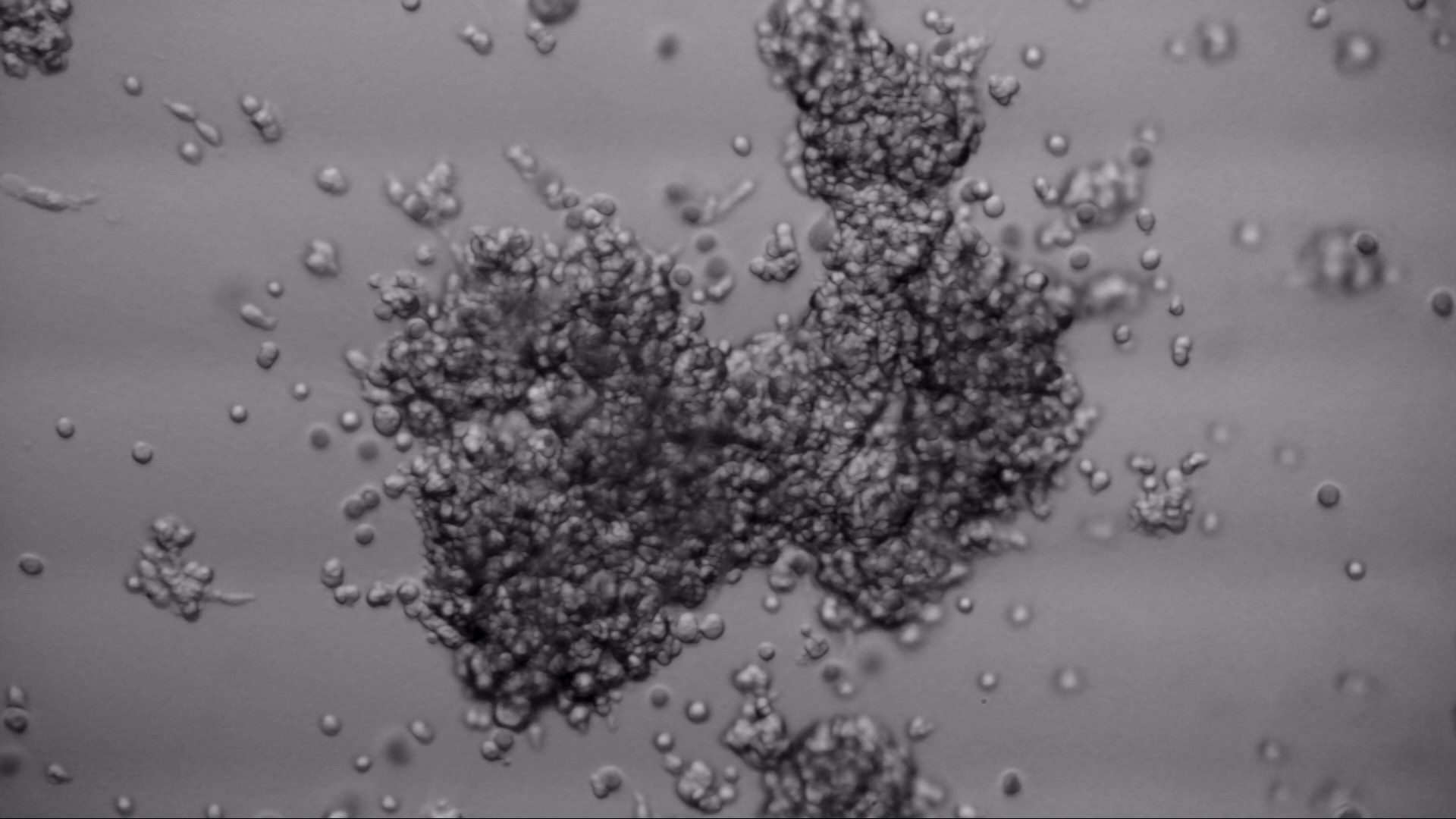
CFPAC-1
CFPAC-1 cells have been cultured on 96-well plate with LifeGel for 14 days.
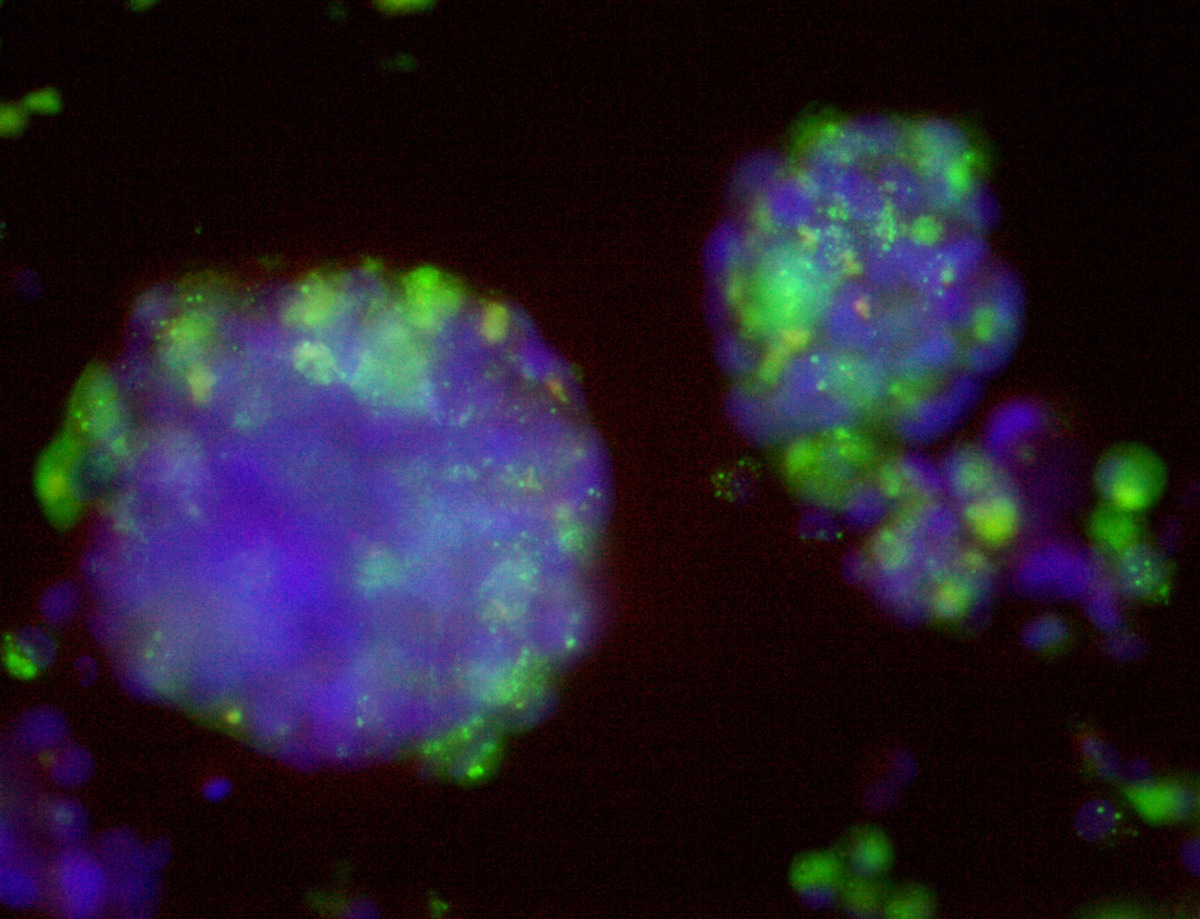
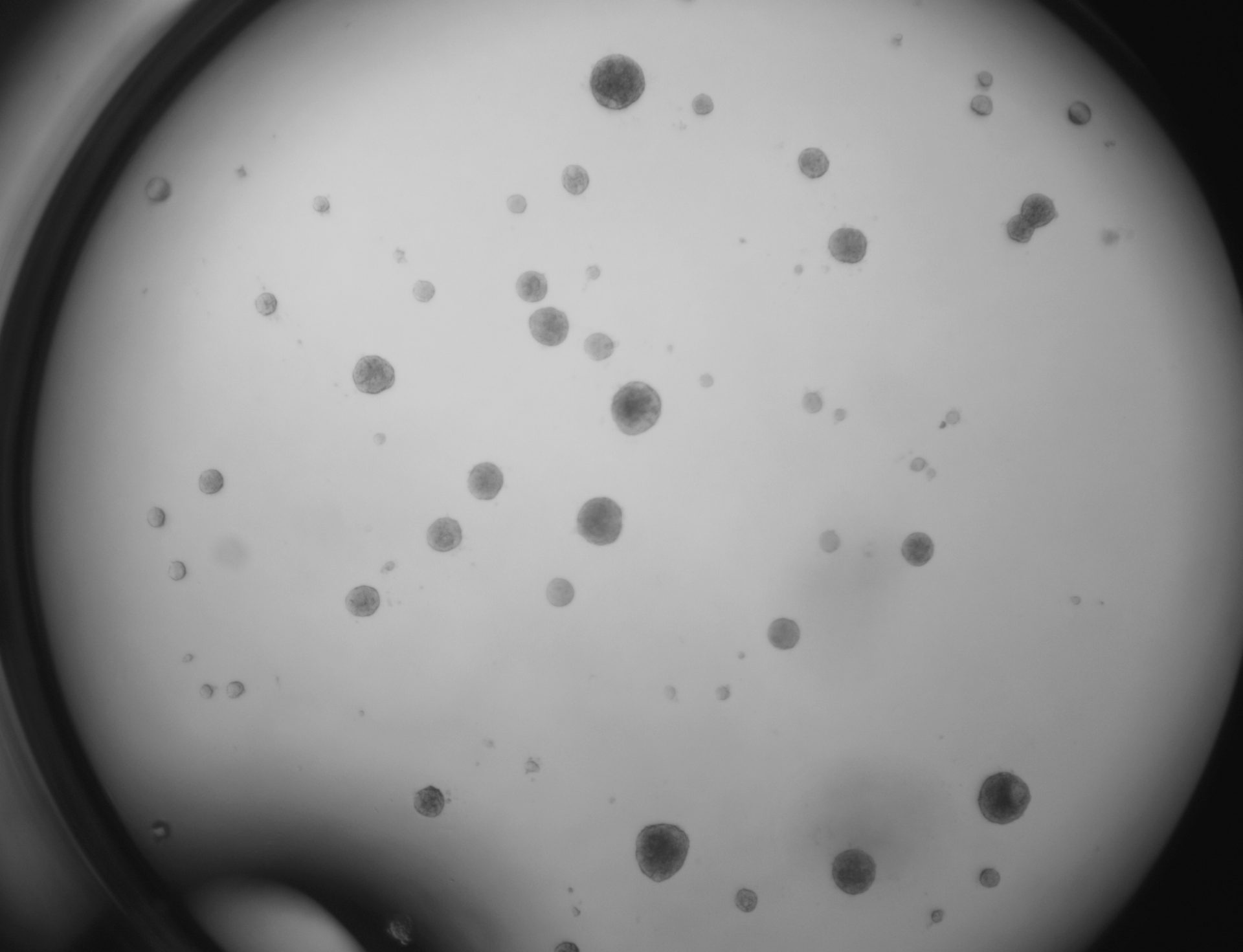
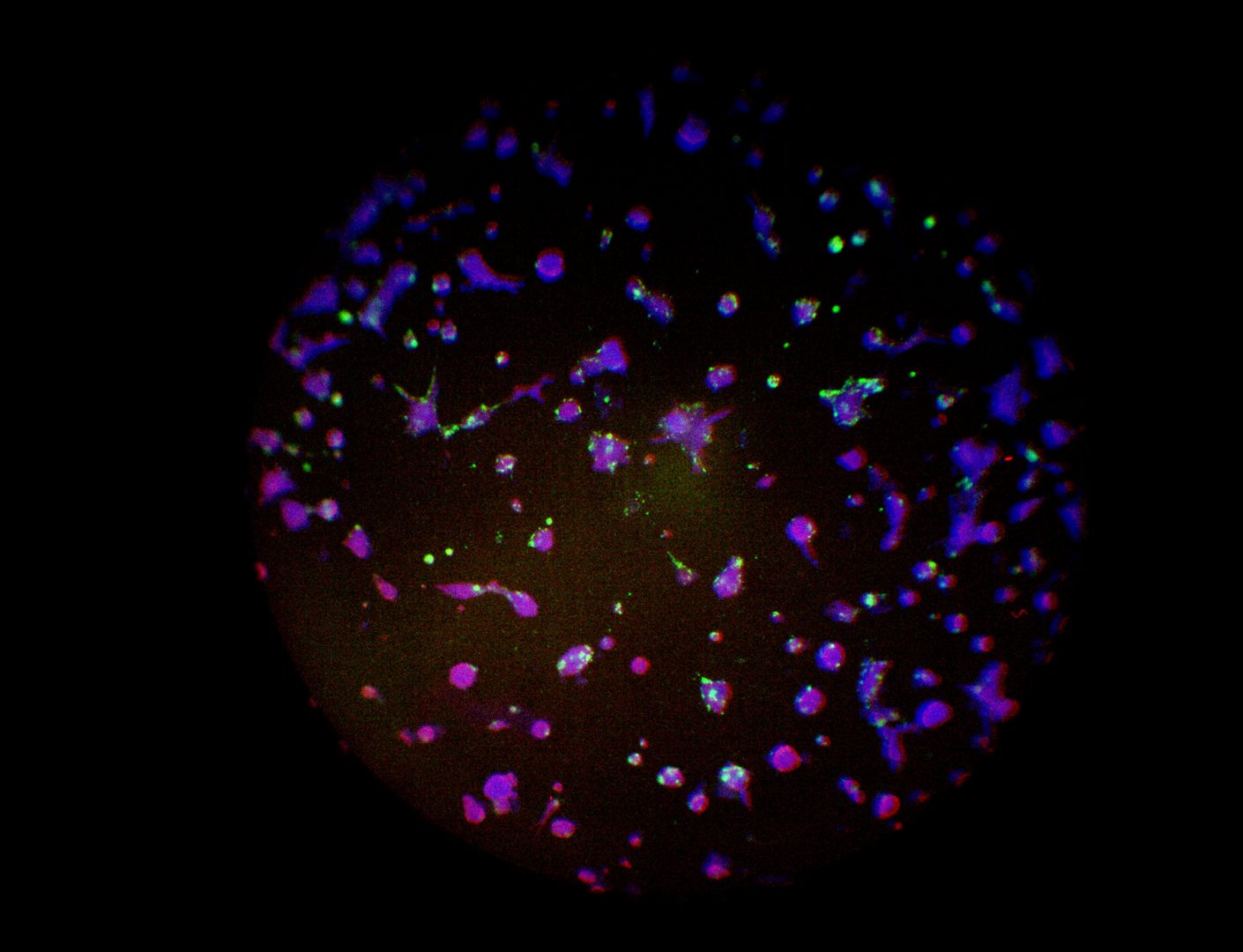
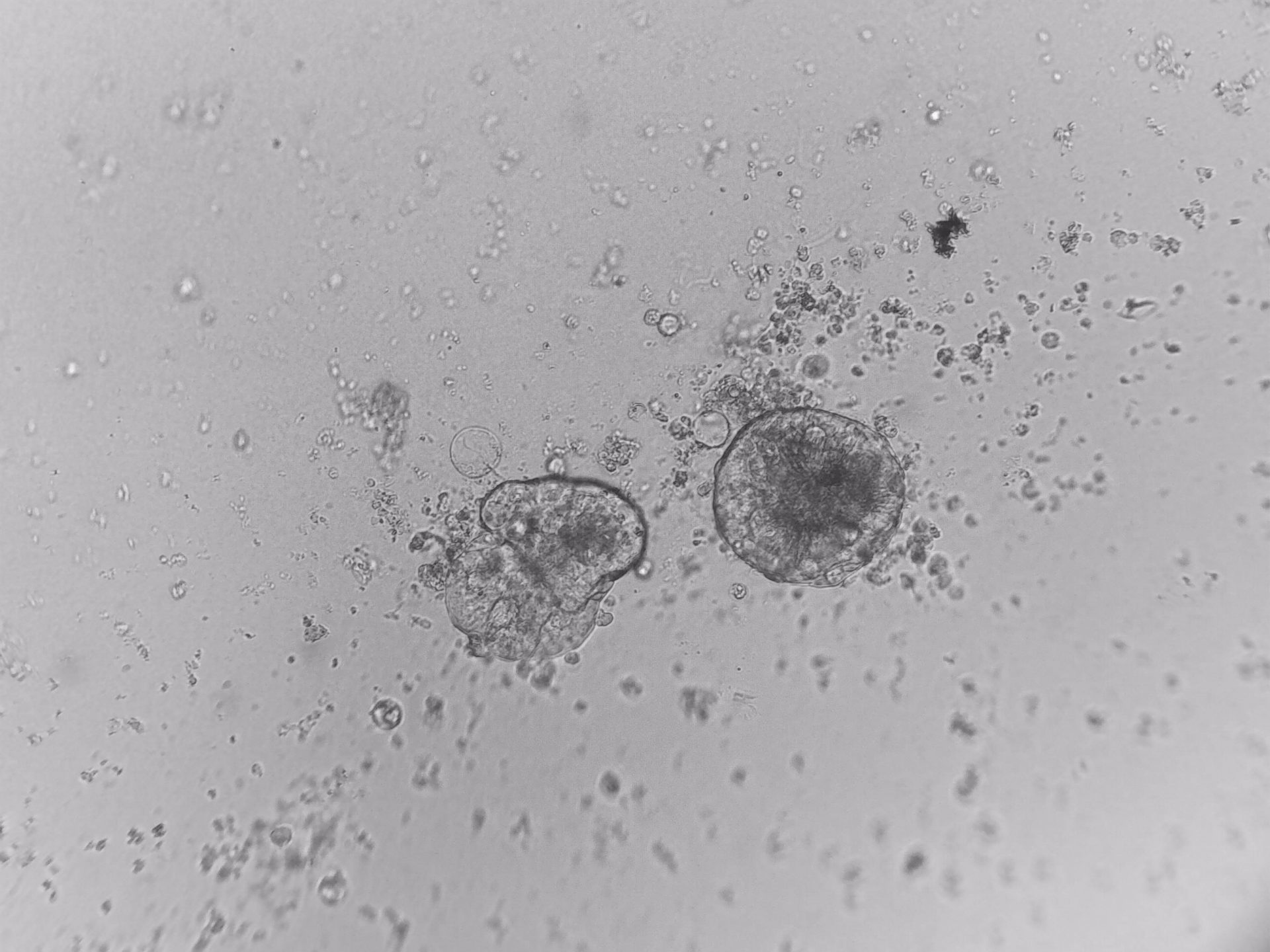
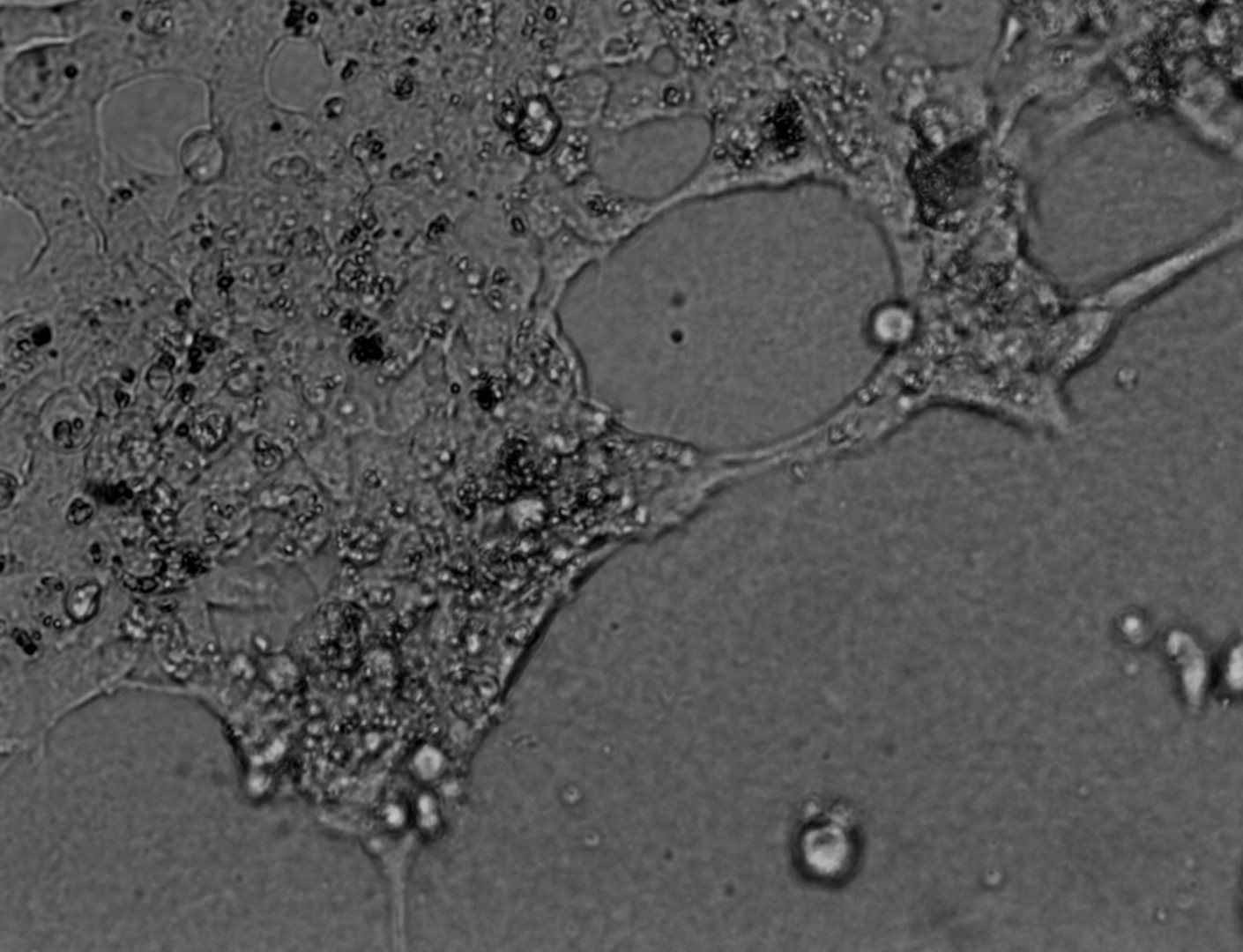
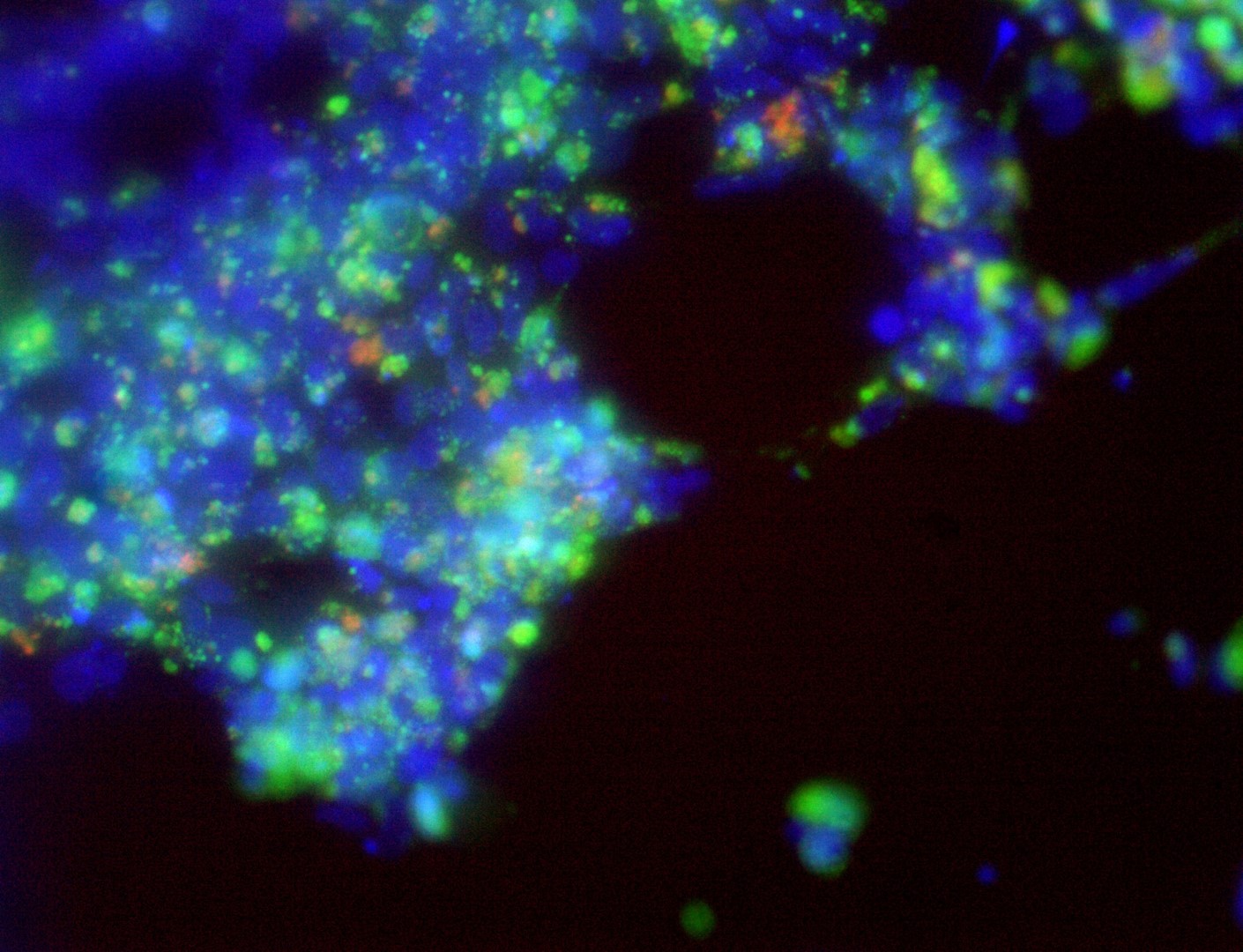
Pan_02
pancreatic ductal adenocarcinoma
Pan_02 cells 3D structures immunofluorescence staining of actin (green), tubulin (red) and nuclei (blue) growing on LifeGel.
Cells were fixed using 4% formaldehyde solution (incubation 10-30 minutes), then perforated with 0,1% Triton-X in 10% FBS (in PBS). Actin was stained using DY-490-Phalloidin from Dynomics (1:500 in PBS). Tubulin was stained using primary (Monoclonal anti-alpha-Tubulin antibody, mouse; 1:500) and secondary (Anti-mouse IgG-Atto594; 5ug/mL) antibodies. Nuclei were stained with Hoechst 33342 (10 ug/mL). Photos: obj. 10x lens 1x.
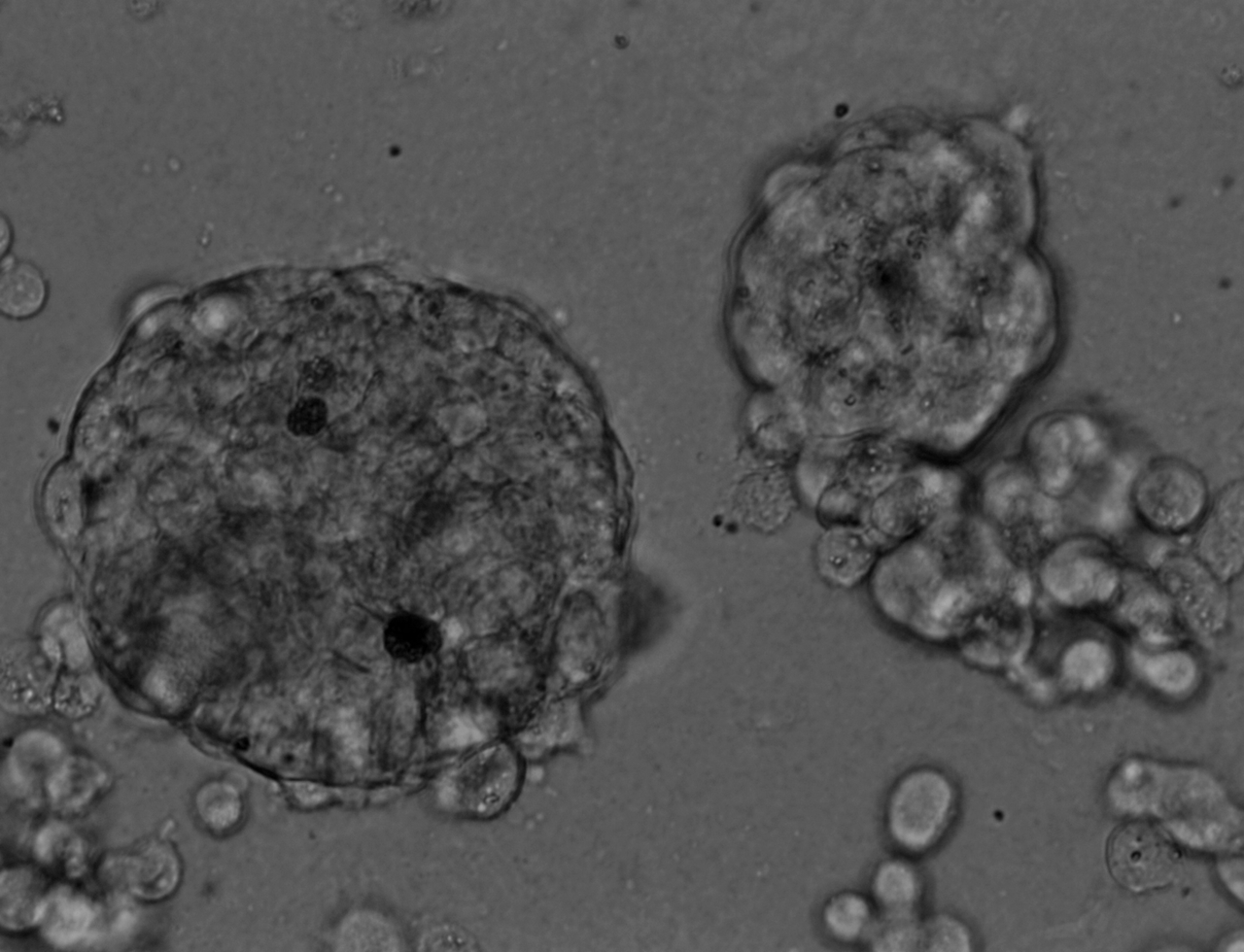
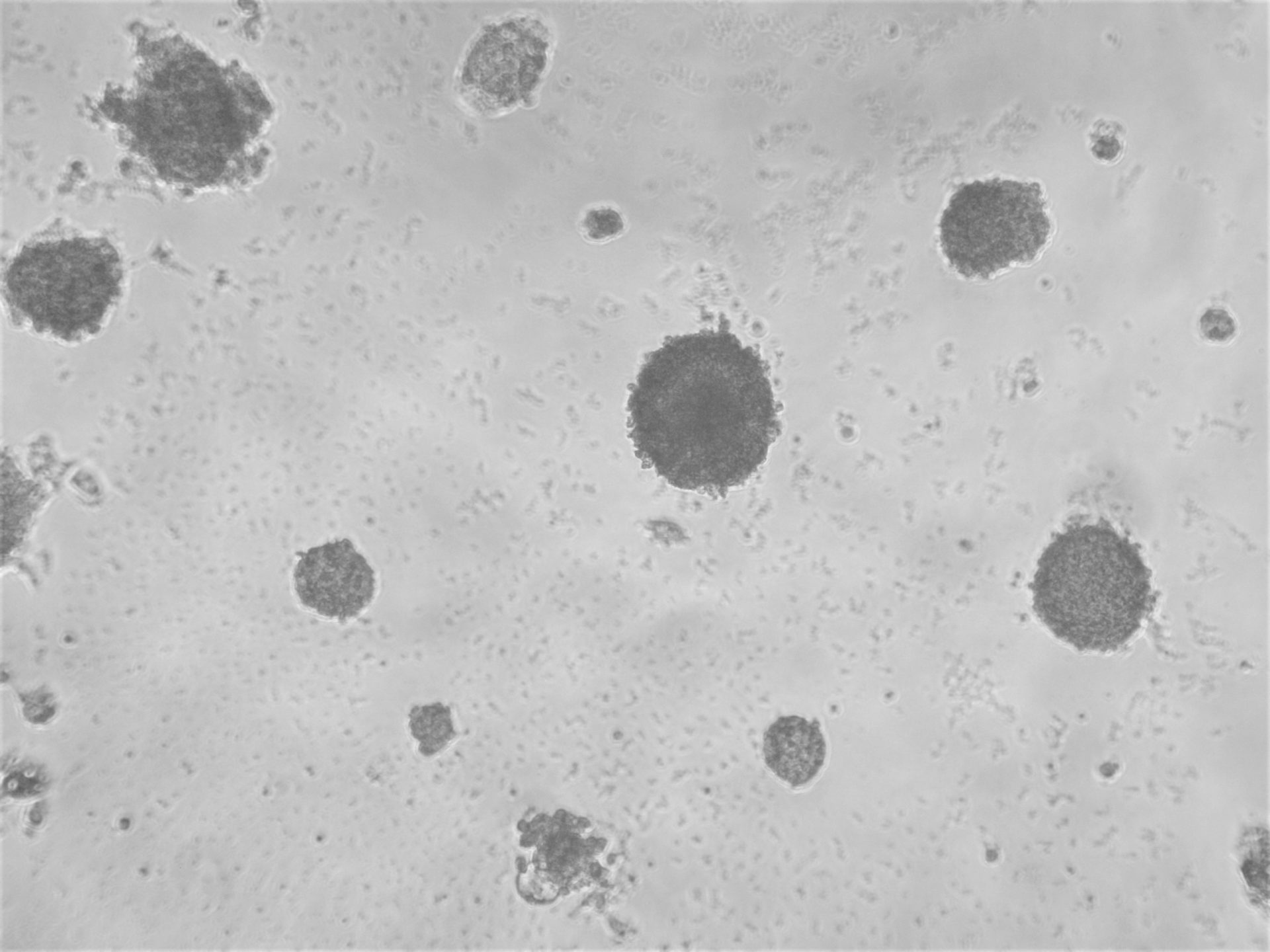
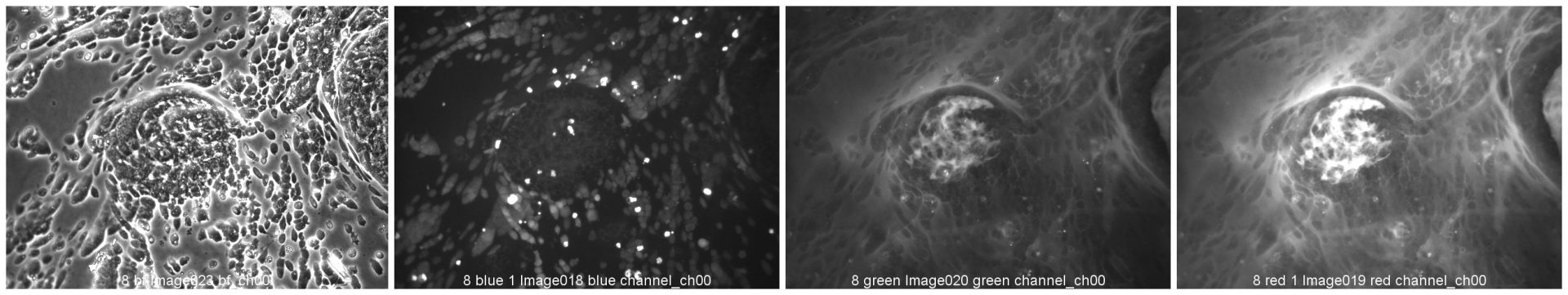
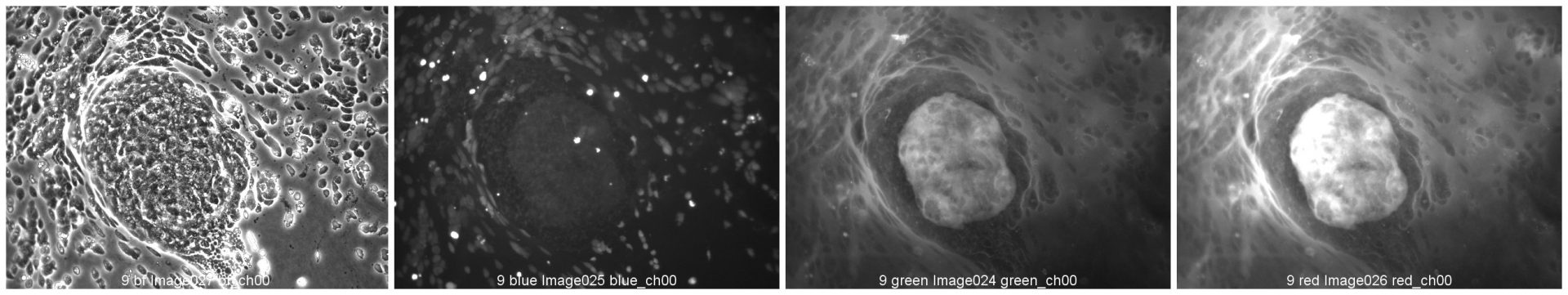
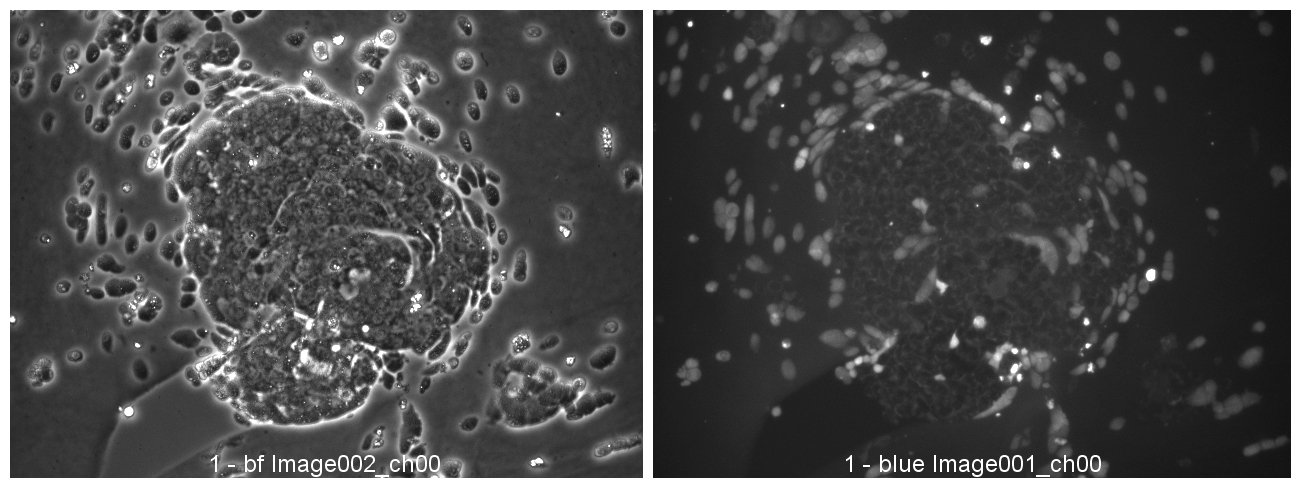
Colorectal and colon cell lines
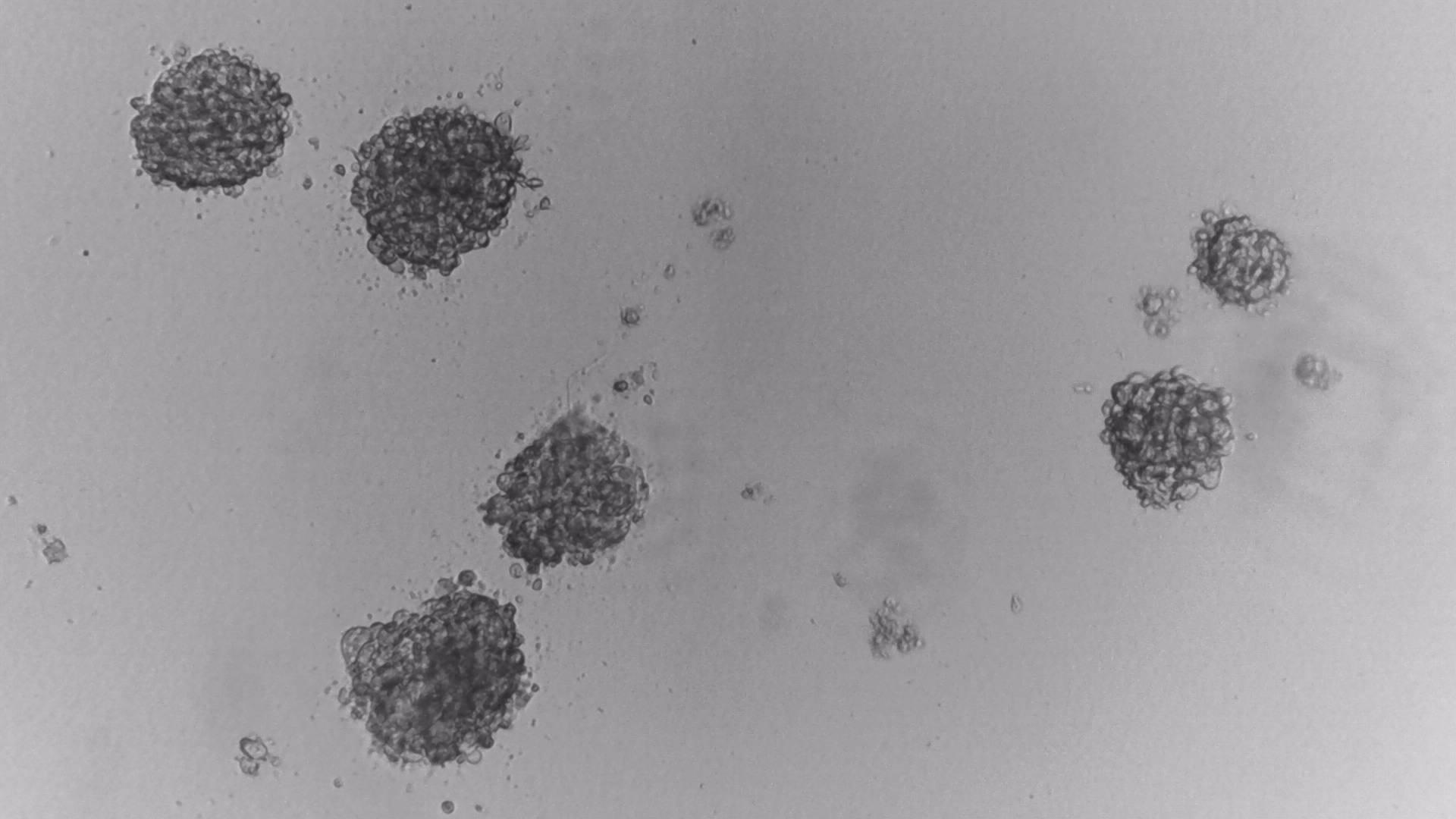
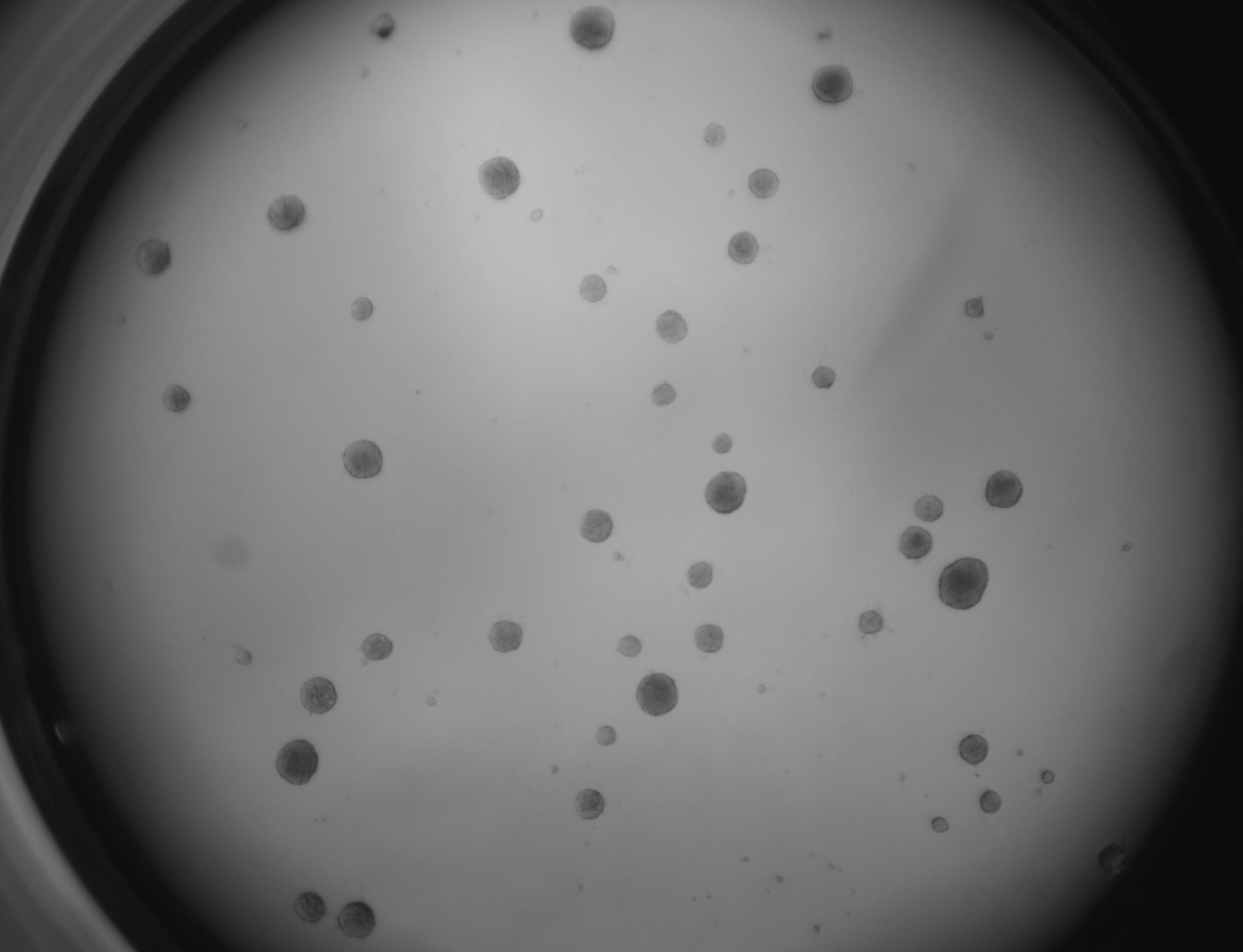
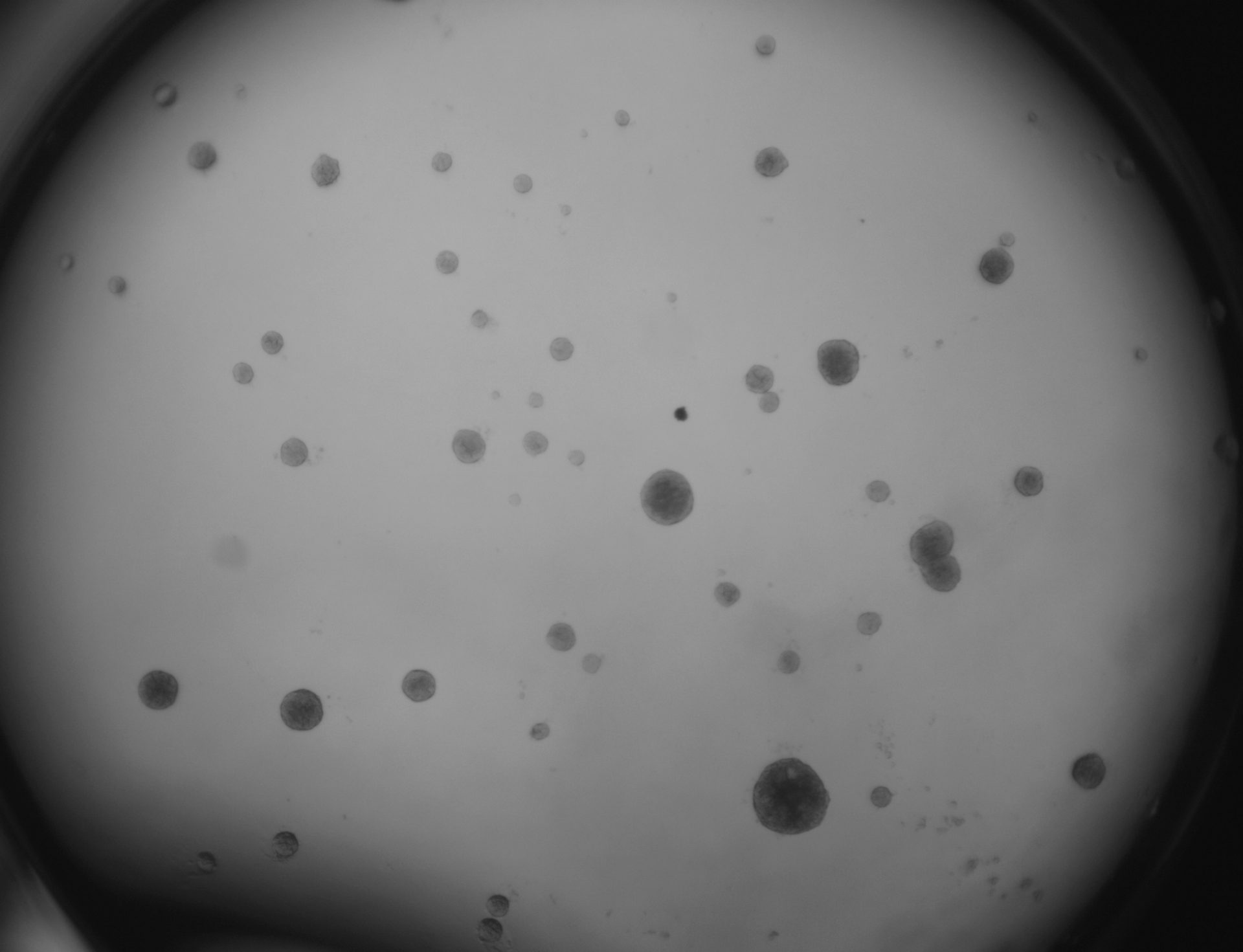
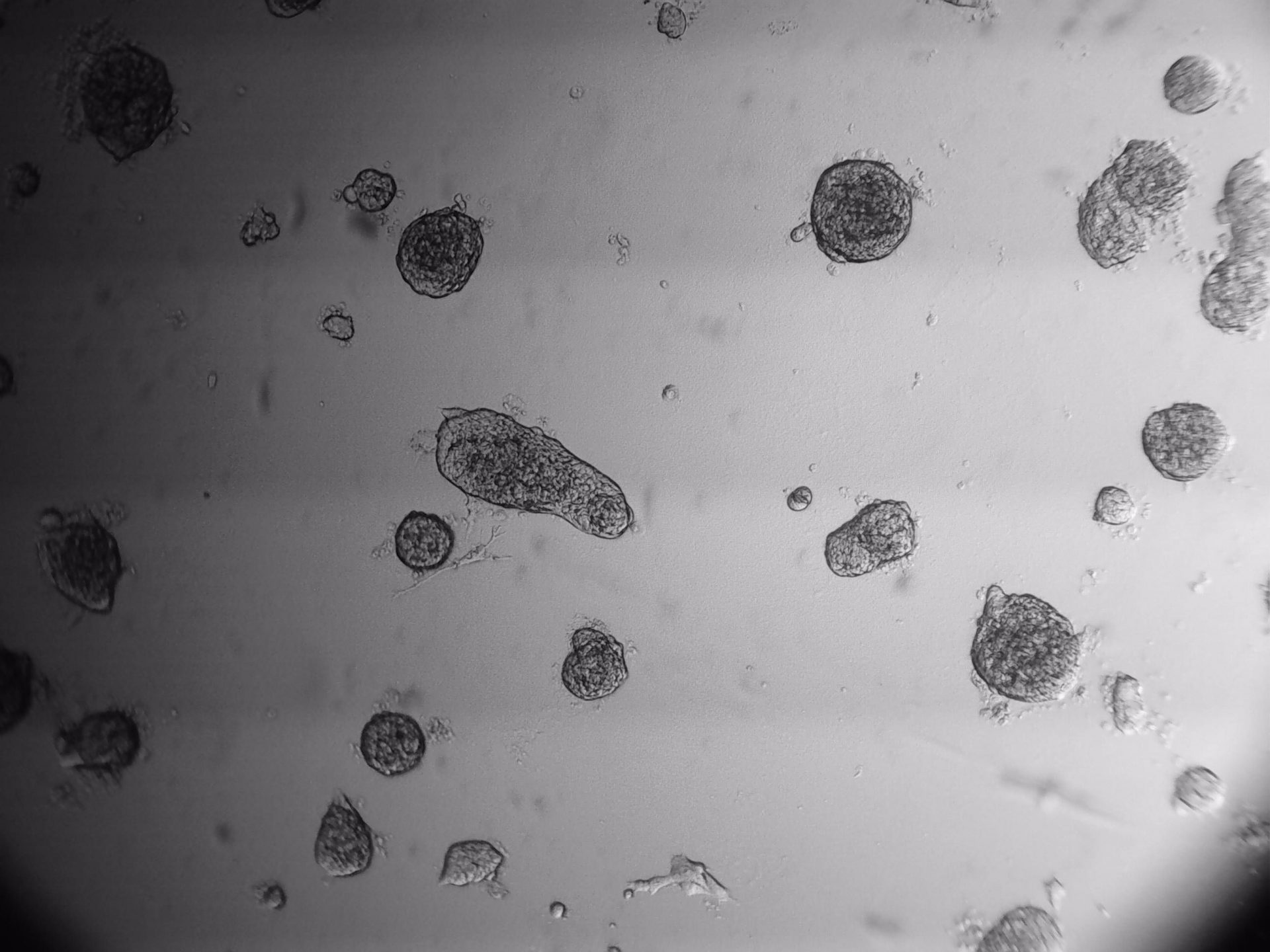
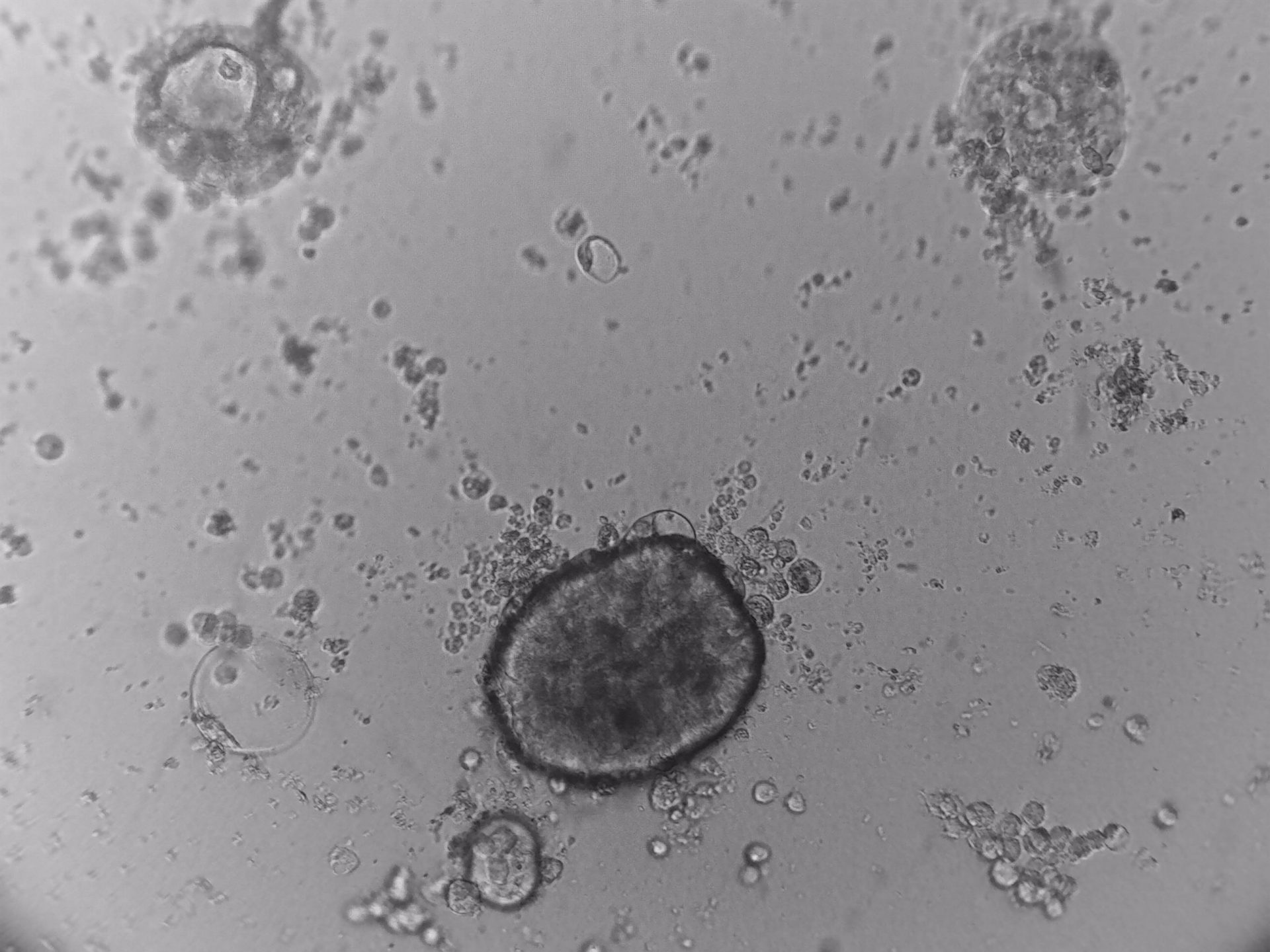
LoVo
LS180
RKO
RKO cells have been cultured on 96-well LifeGel plates for 12 days, they have been seeded 1500 cells/well.
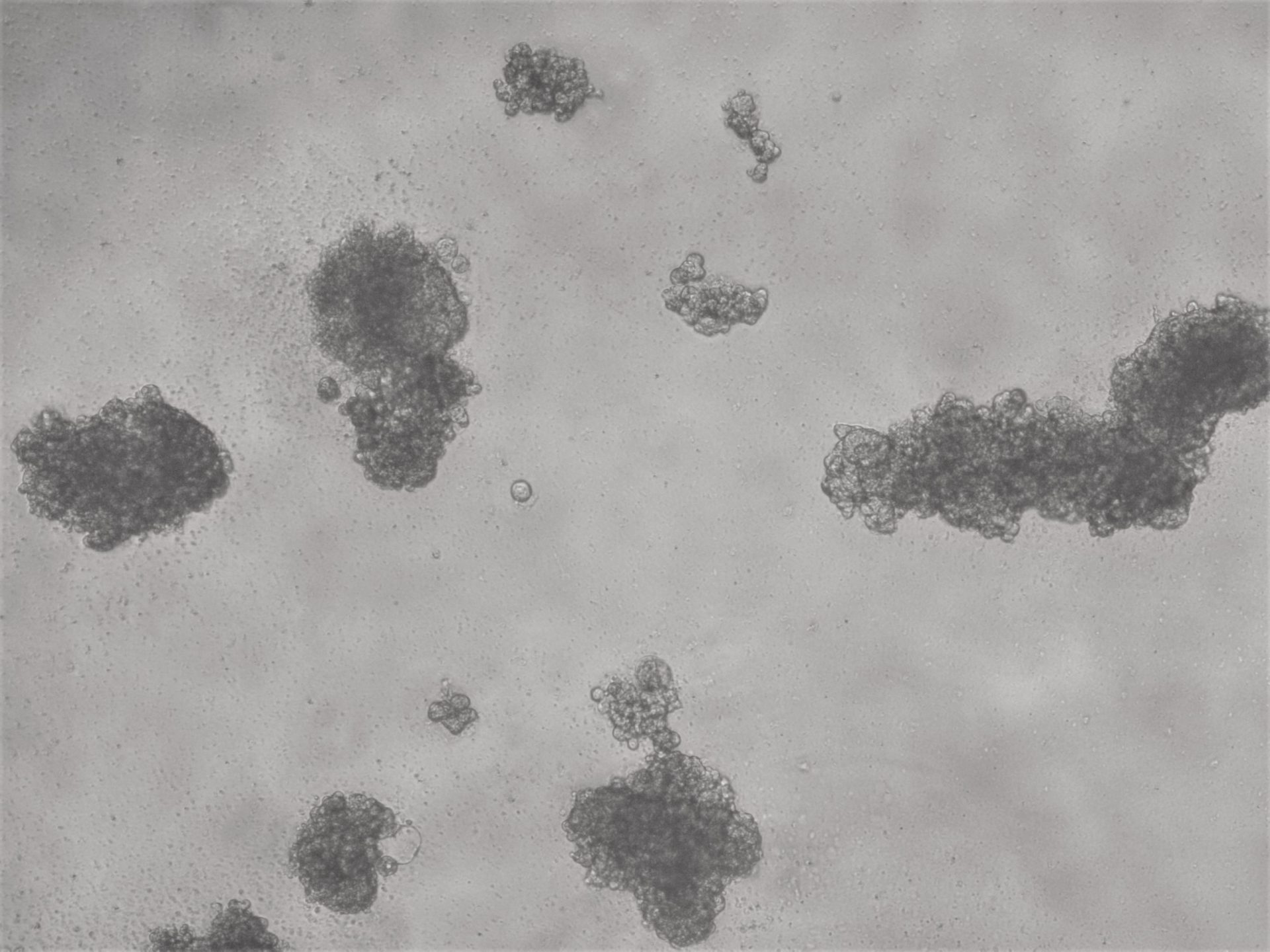
Breast cancer cell lines
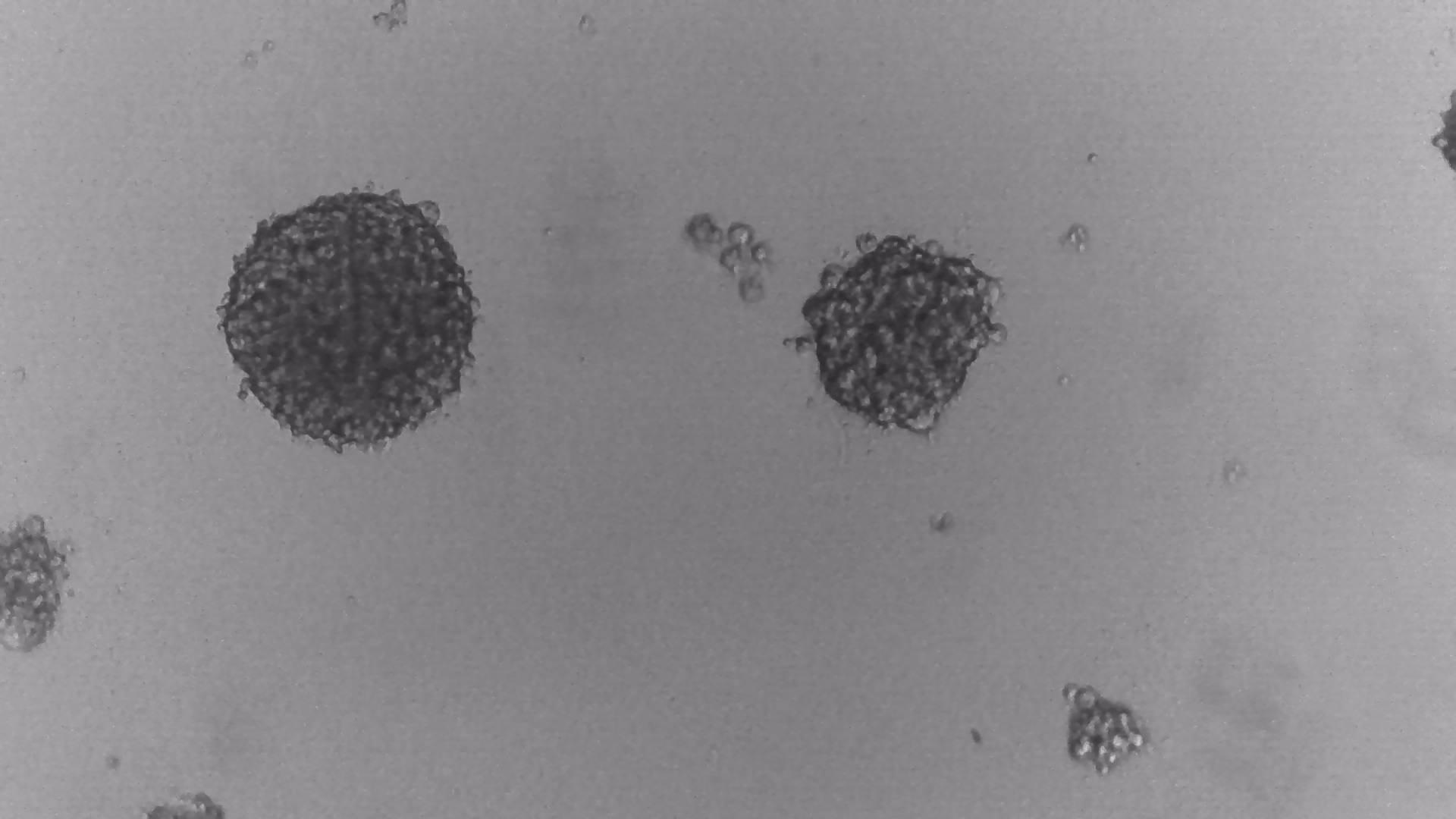
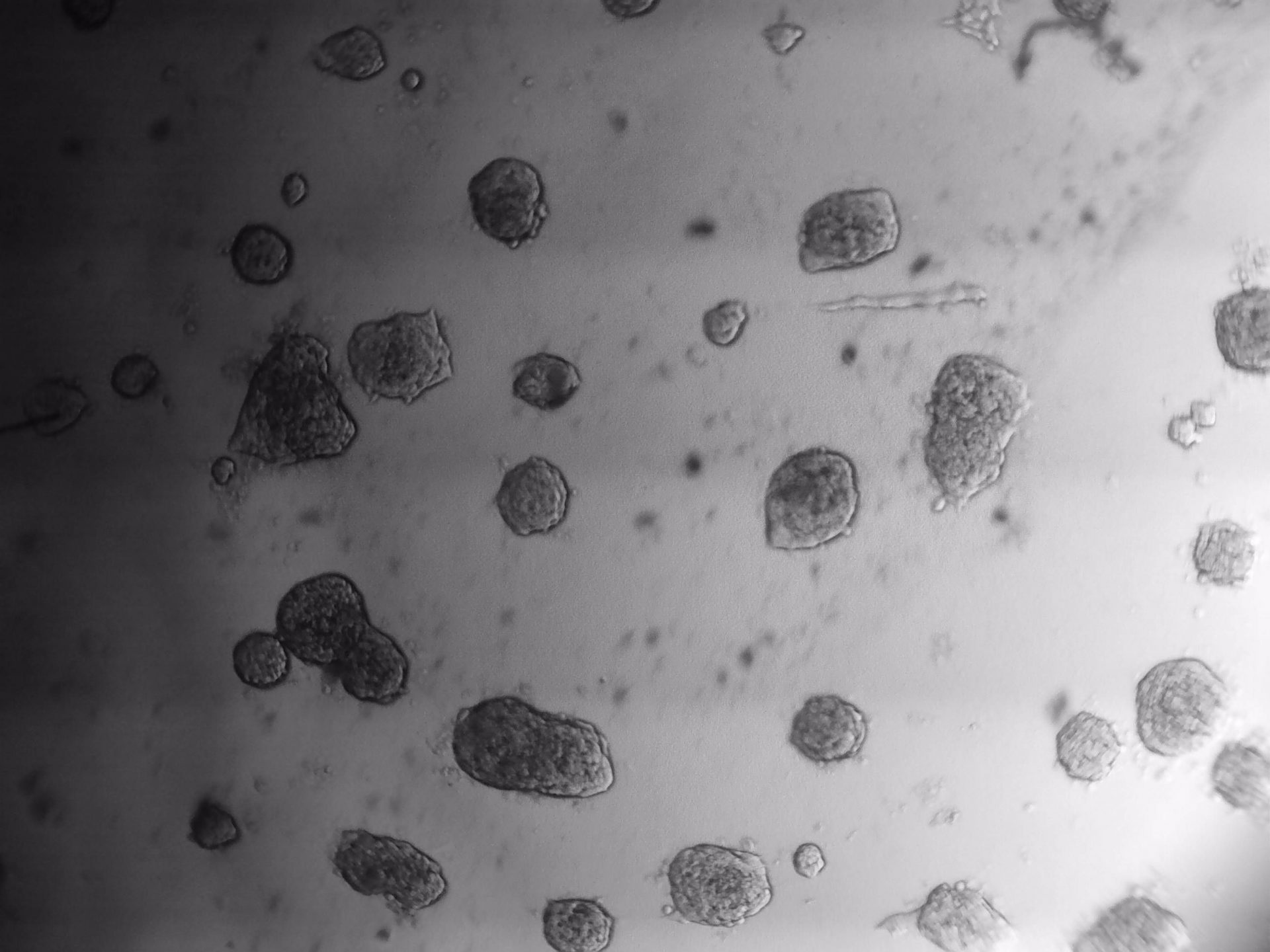
MDA-MB-231
MDA-MB-231 cells have been cultured on LifeGel for 10 days.
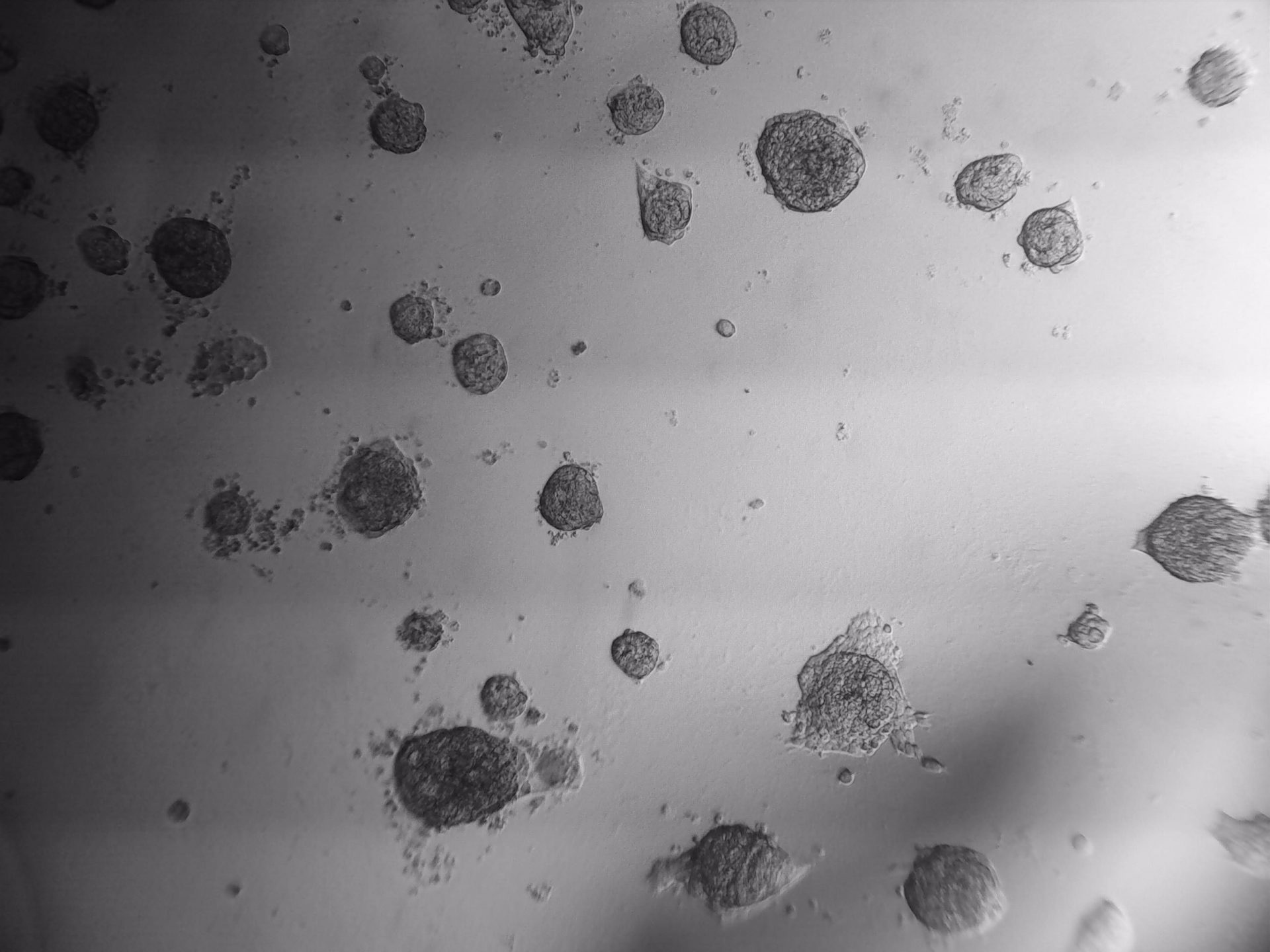
4T1
4T1 cells shown below have been cultured on 96-well LifeGel plate for 7 days. Cells were seeded 500 cells/well.
4T1 cells shown below have been cultured on µ-Slide angiogenesis plate with LifeGel for 7 days. Cells were seeded 500 cells/well.
On the photos below there is staining with cultured 4T1 cells on 96-well LifeGel plate. DiO was for cell membranes (green), MitoLite for mitochondria (red), Hoechst for nuclei (blue). Obj. 2x, lens 1x.
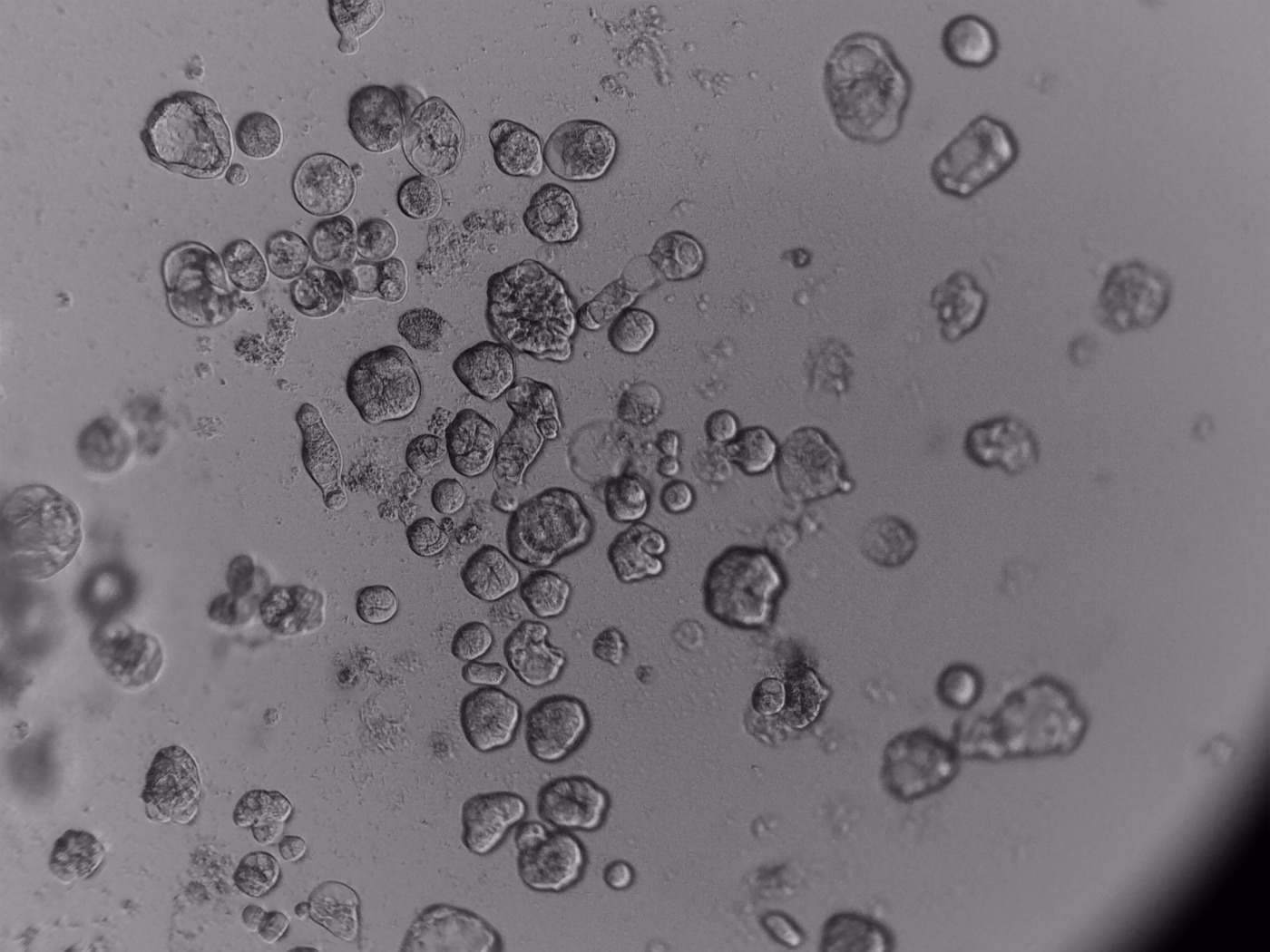
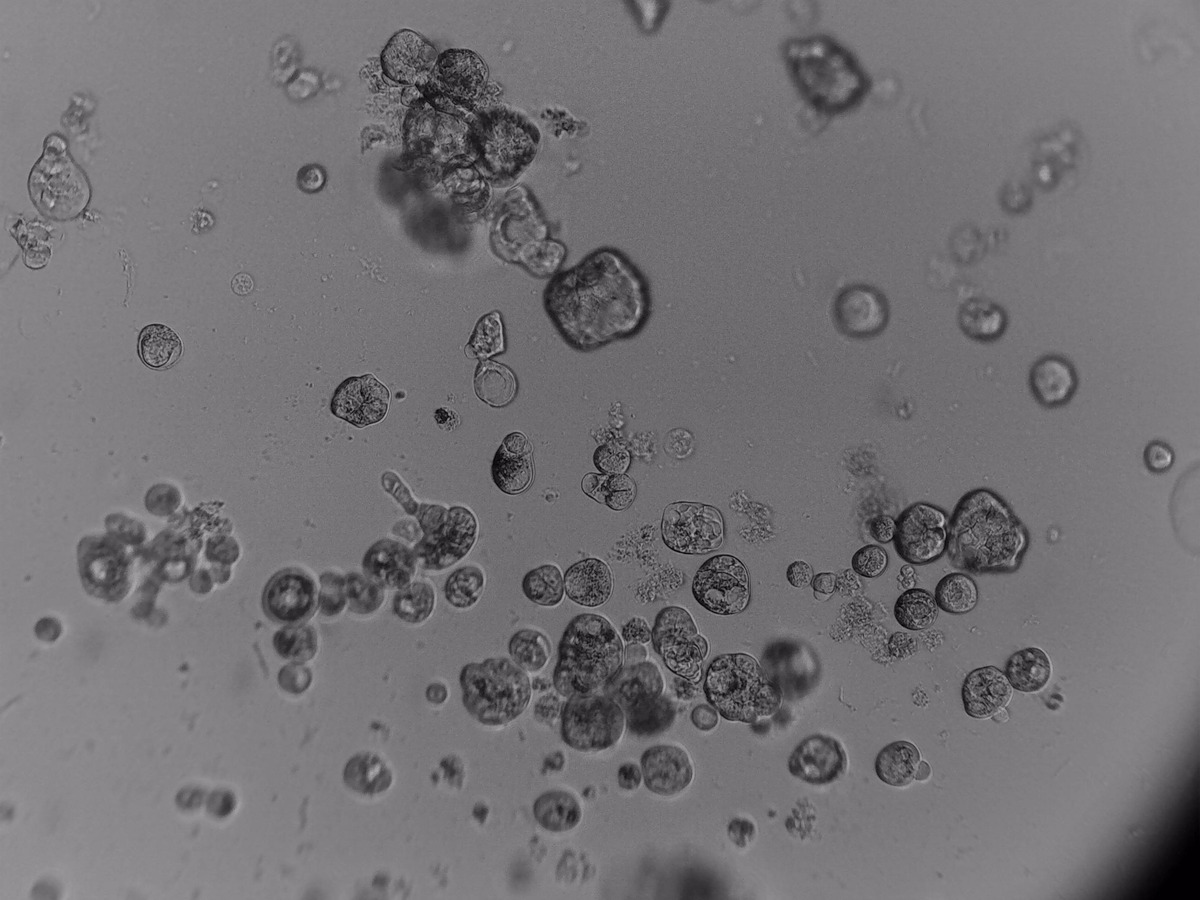
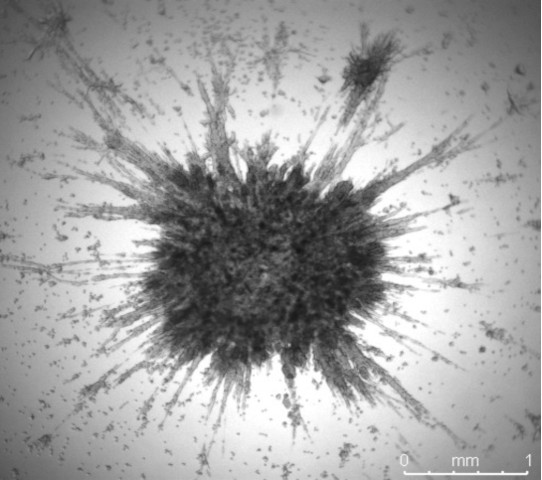
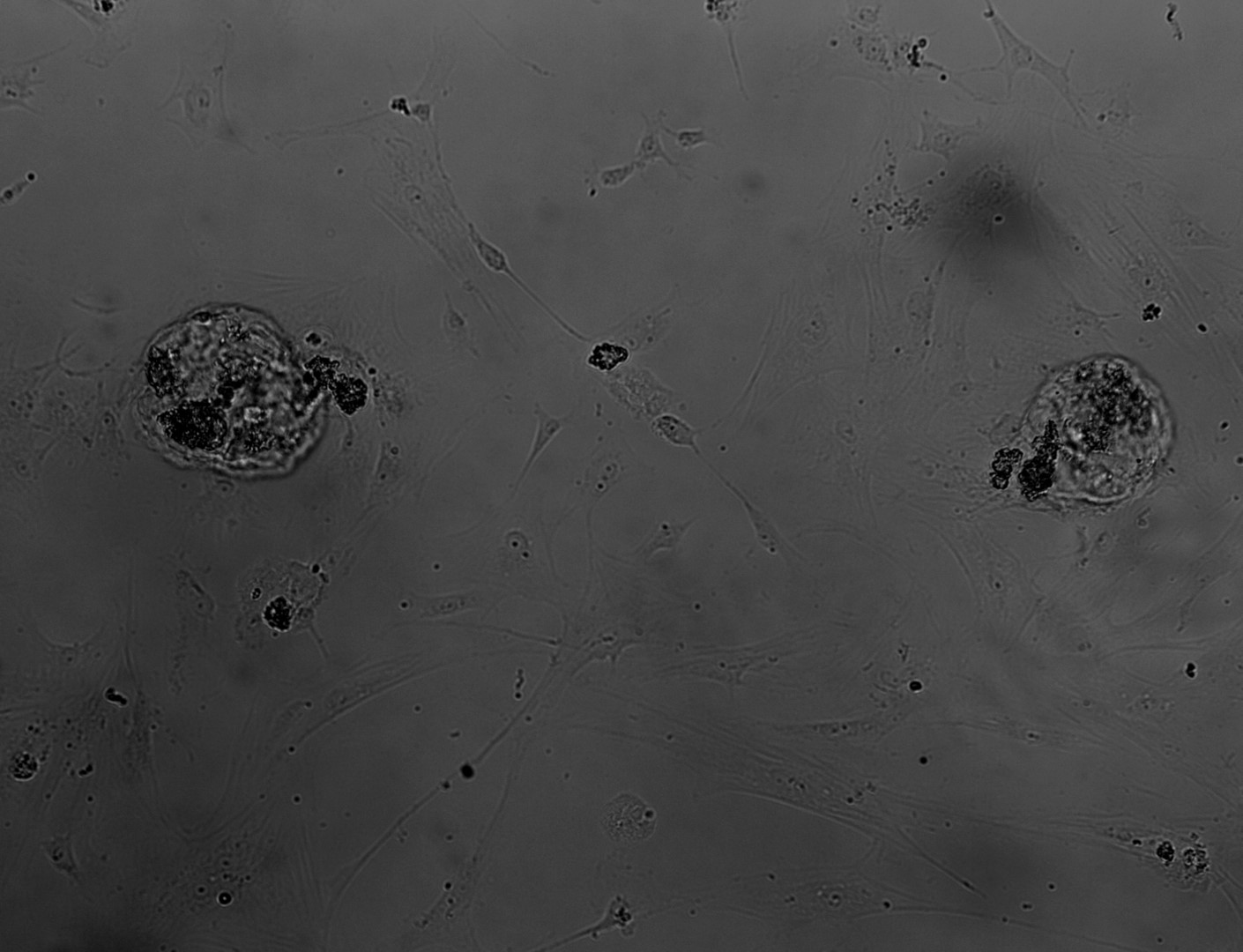
PDX (Patient Derived Xenograft)
PDX (Patient Derived Xenograft)
primary cells
3D ex vivo culture of patient-derived xenograft model of colorectal cancer. Tumor sample was dissociated and cultured on 96-well plate with LifeGel for 14 days. Microscope images were taken using ZEISS Telaval 31 Inverted Microscope at 10x objective.
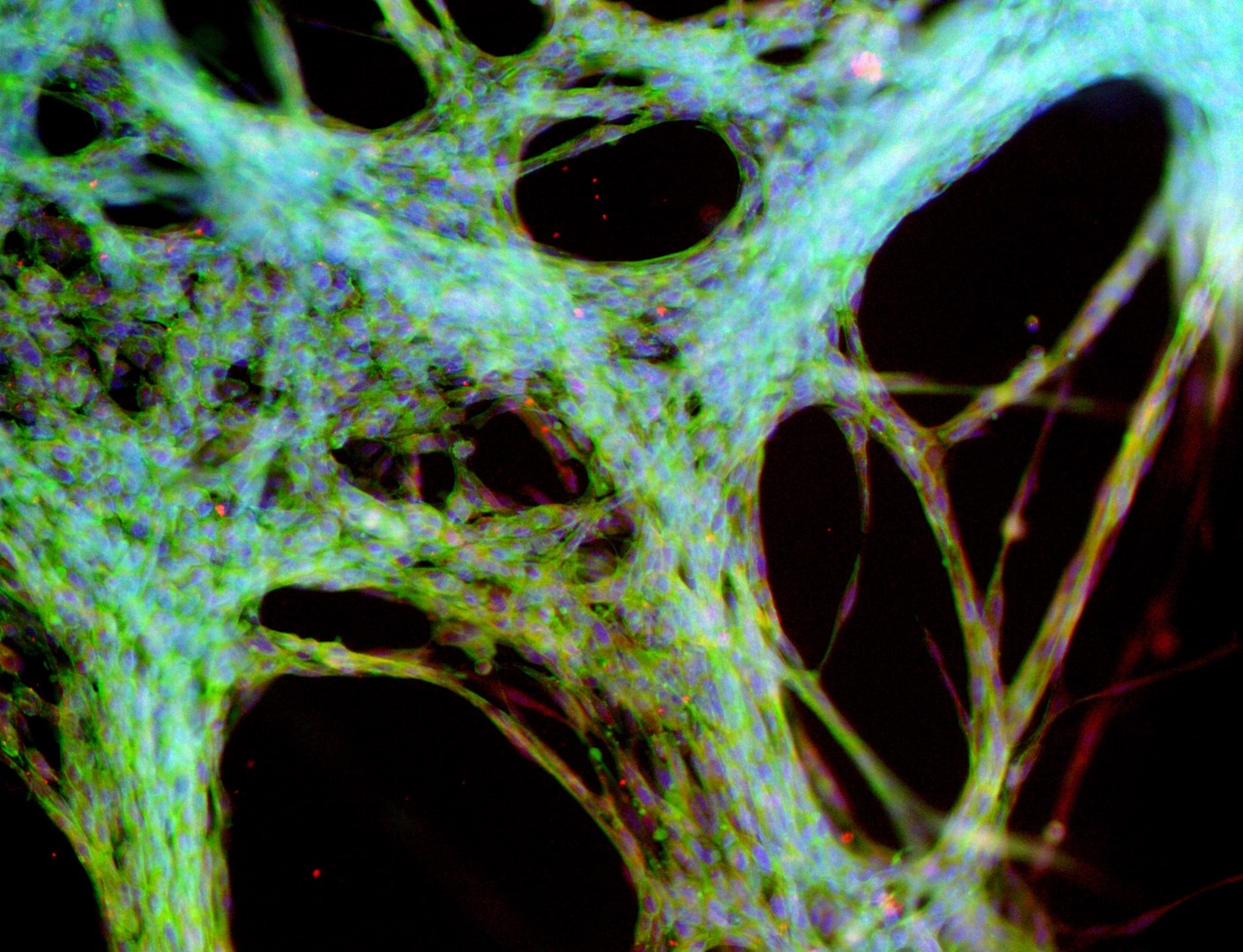
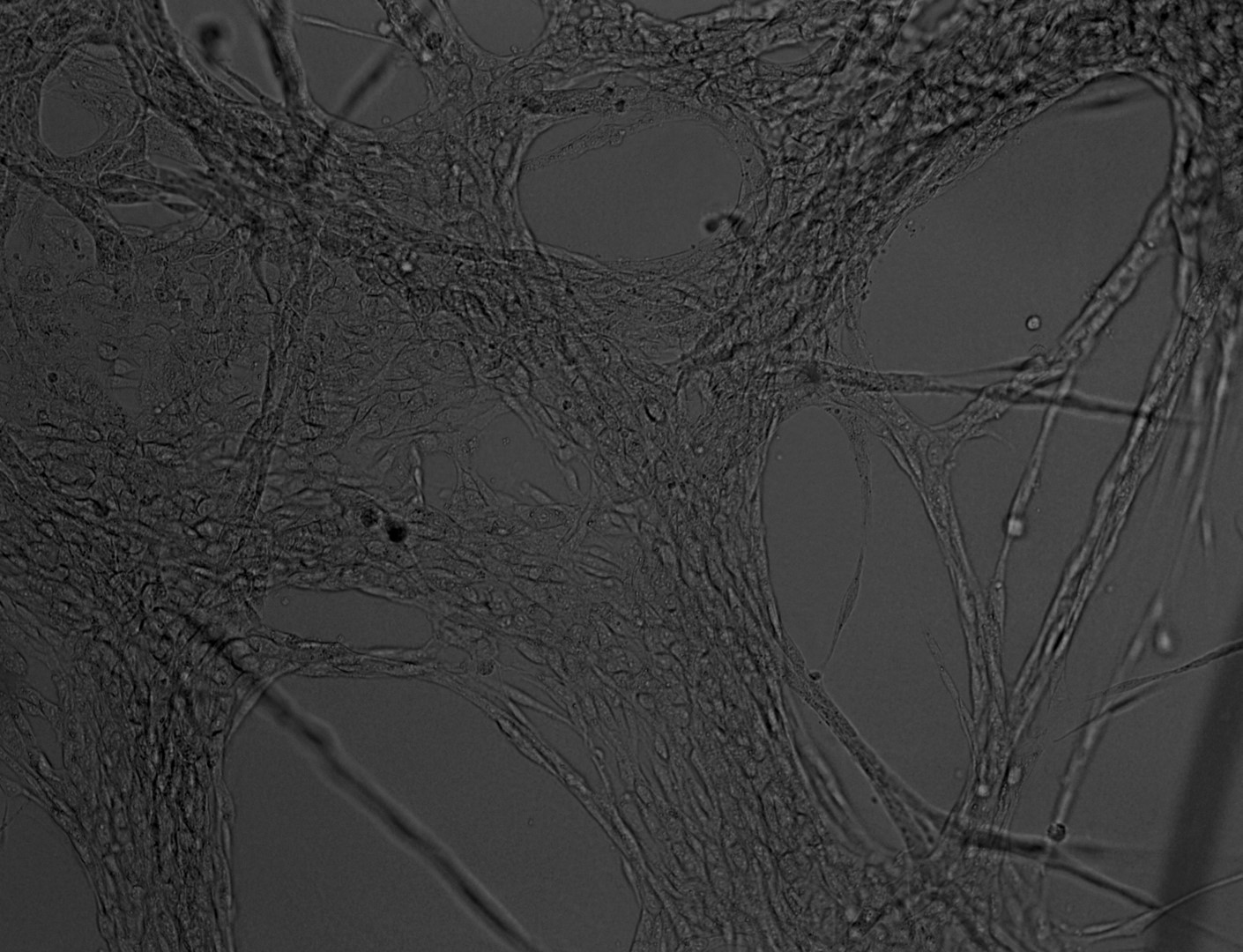
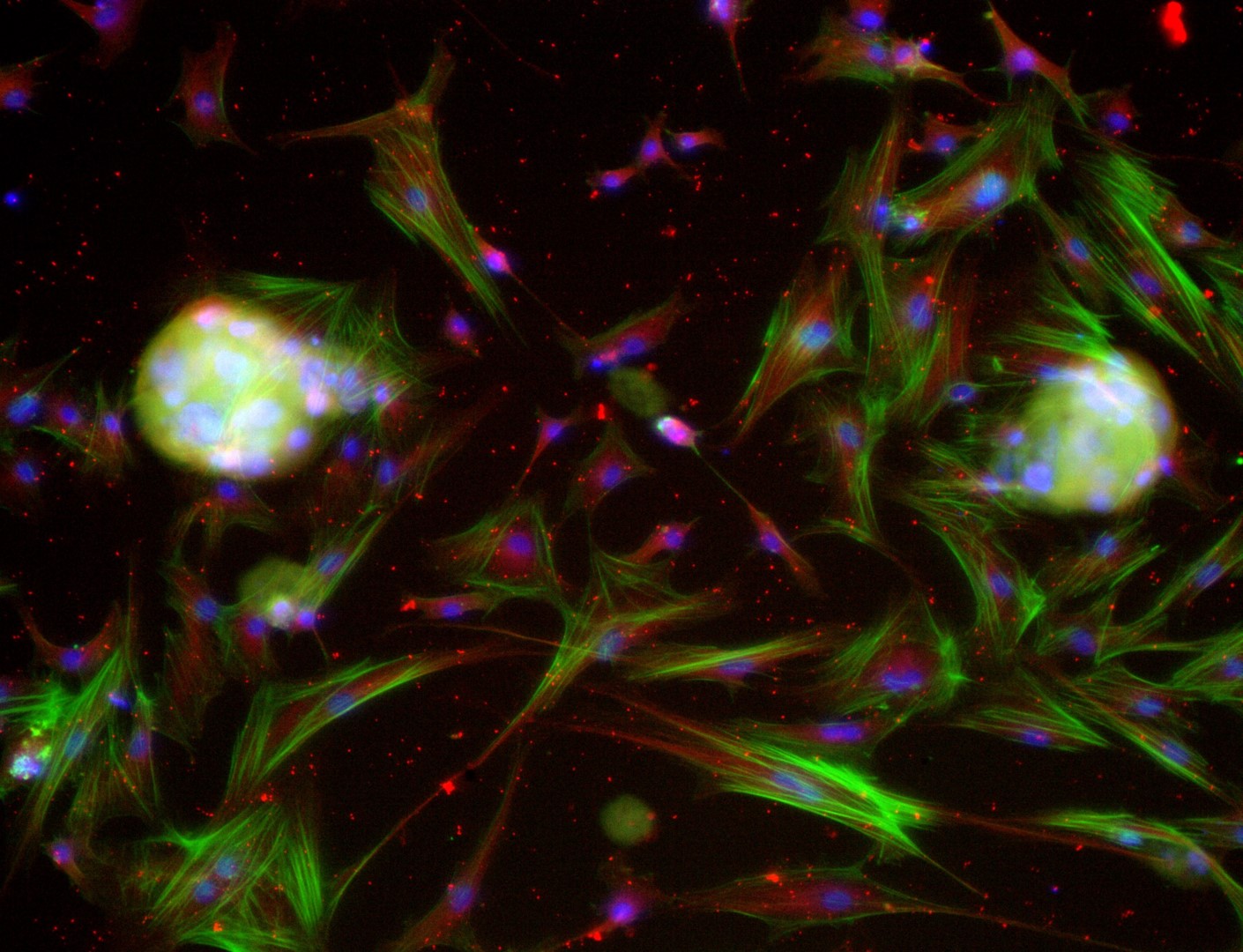
Mesenchymal stem cells
MSC
mesenchymal stem cells
Mesenchymal stem cells culture on LifeGel have been stained for actin (green), tubulin (red) and nuclei (blue).
Cells were fixed using 4% formaldehyde solution (incubation 10-30 minutes), then perforated with 0,1% Triton-X in 10% FBS (in PBS). Actin was stained using DY-490-Phalloidin from Dynomics (1:500 in PBS). Tubulin was stained using primary (Monoclonal anti-alpha-Tubulin antibody, mouse; 1:500) and secondary (Anti-mouse IgG-Atto594; 5ug/mL) antibodies. Nuclei were stained with Hoechst 33342 (10 ug/mL). Photos: obj. 10x lens 1x.
Other cell lines
RCC
renal cell carcinoma
Cells have been fixed on LifeGel and fluorescently stained with antibodies for: F-actin (green colour), α-tubulin (red colour), nucleus (blue colour). Photos in the second line is with brightfield and darkfield.
Project have been discovered in cooperation with IBIDI. Photos made by Shokoufeh Teymouri, µ-Slide Angiogenesis, IBIDI GmbH.
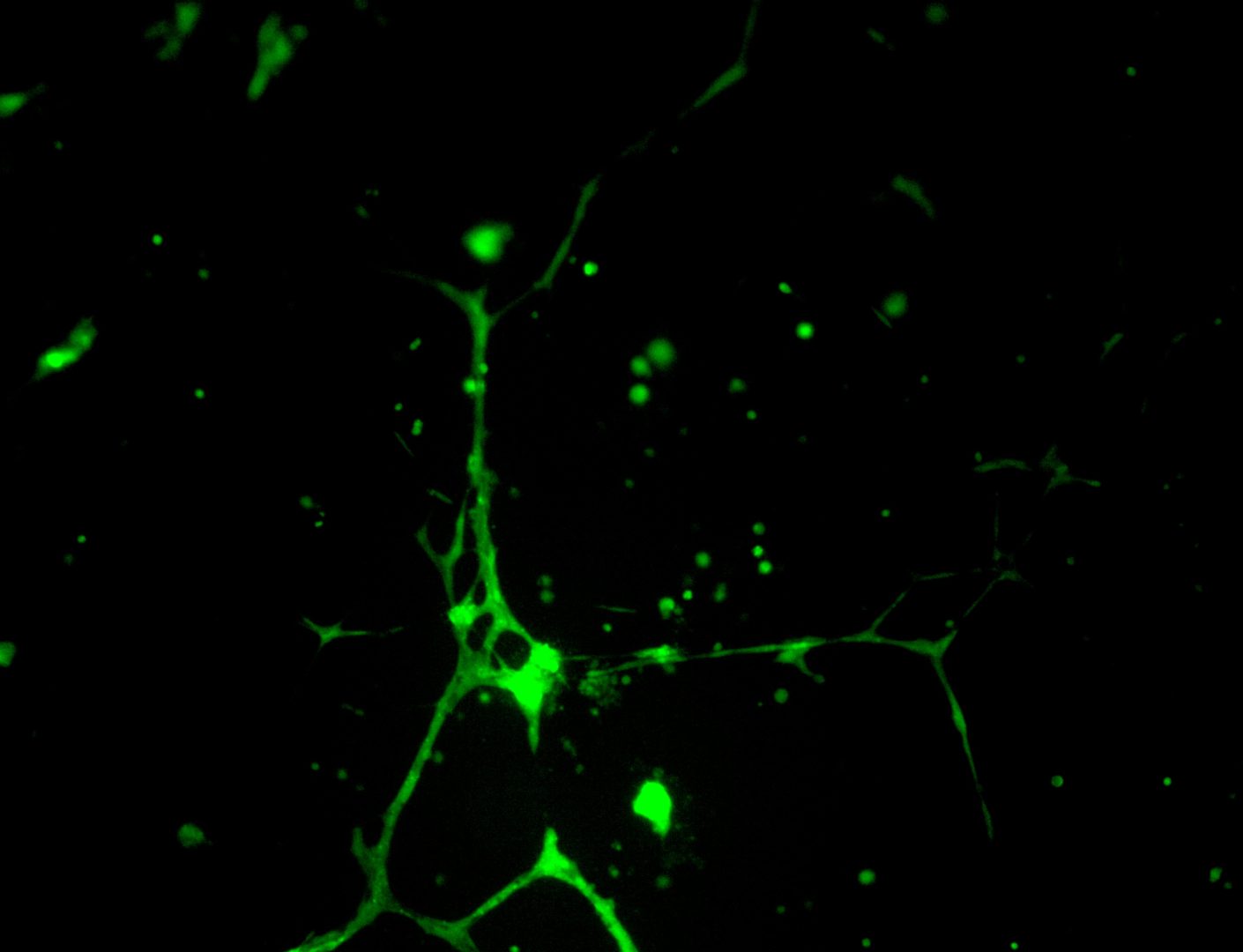
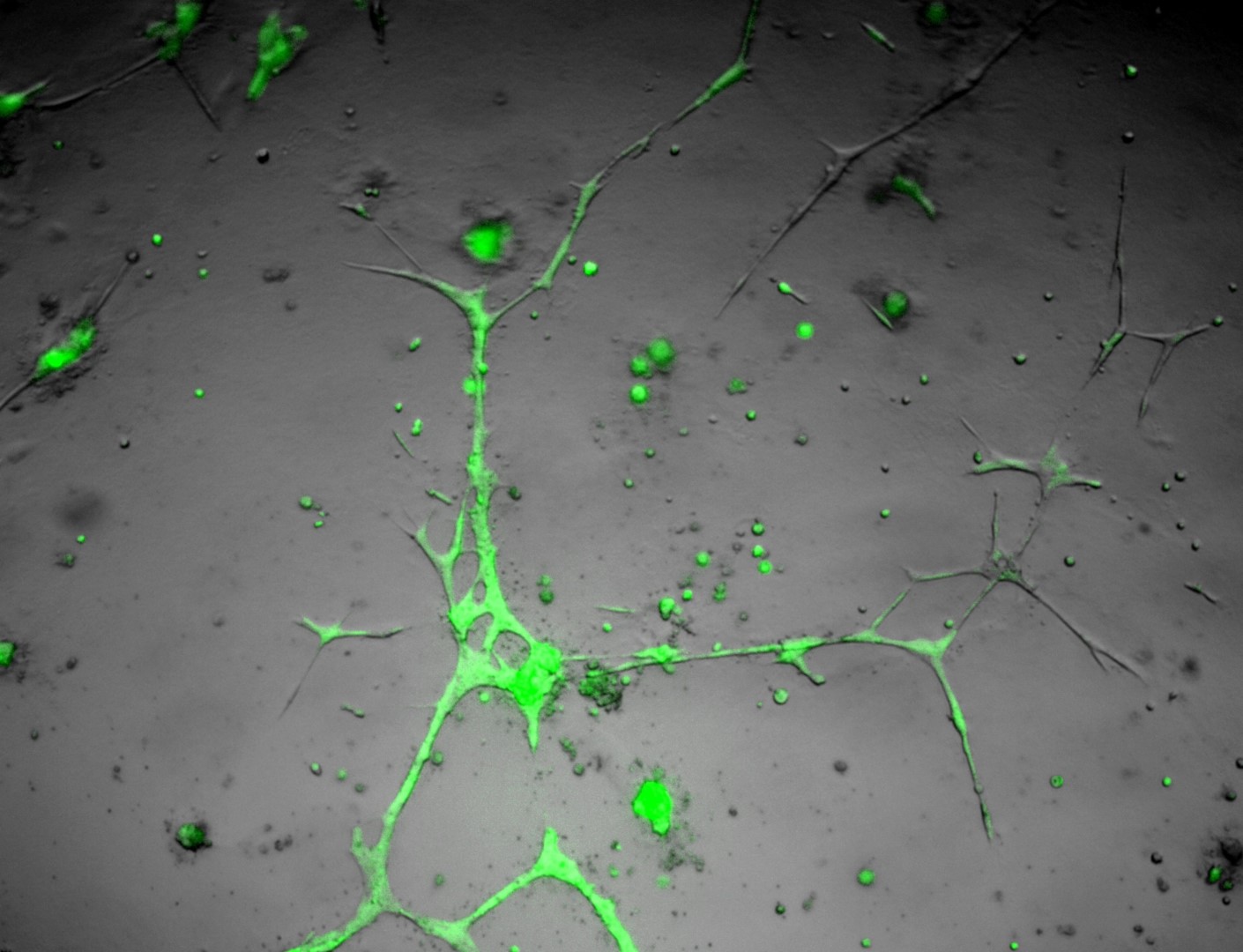
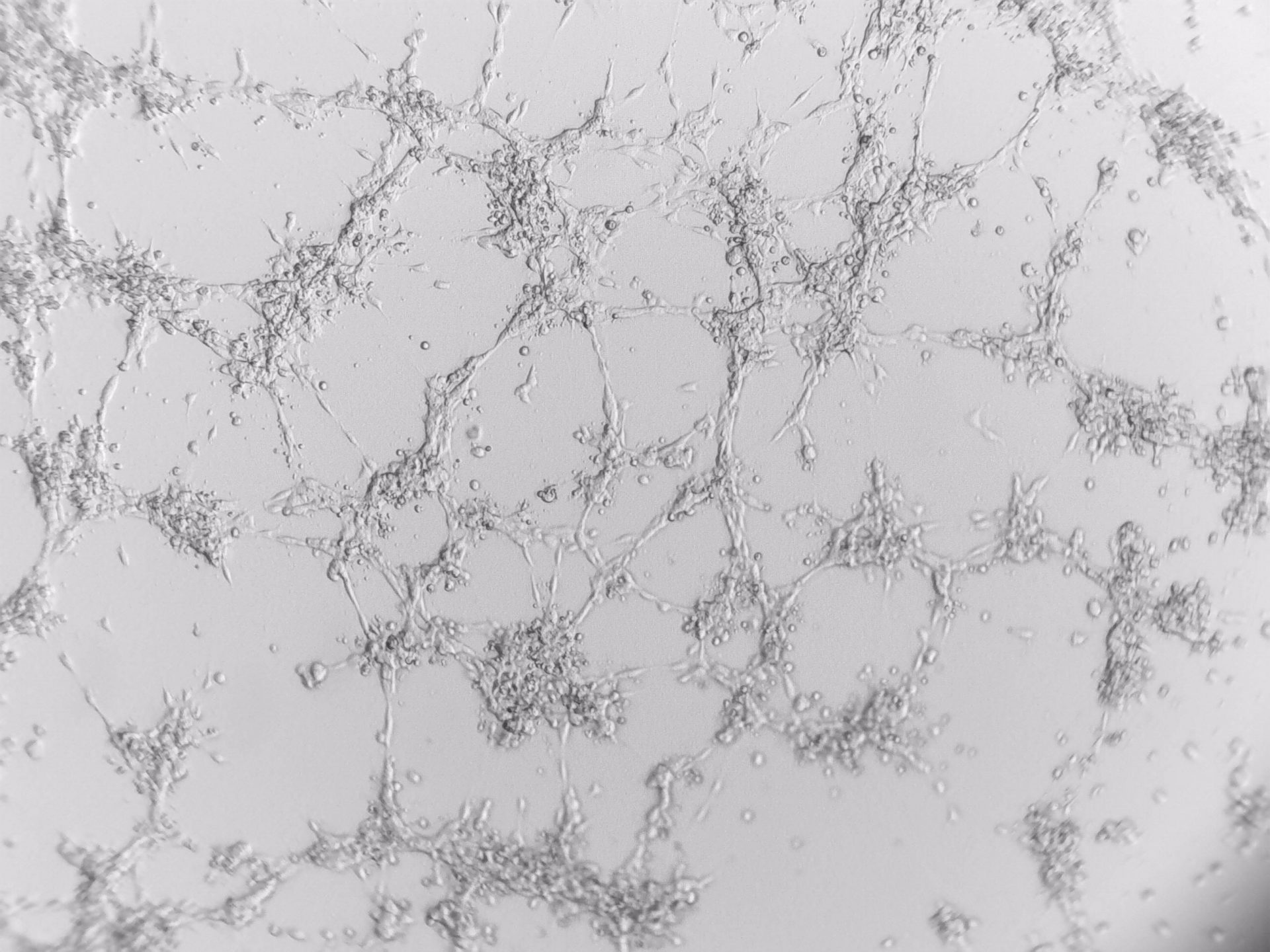
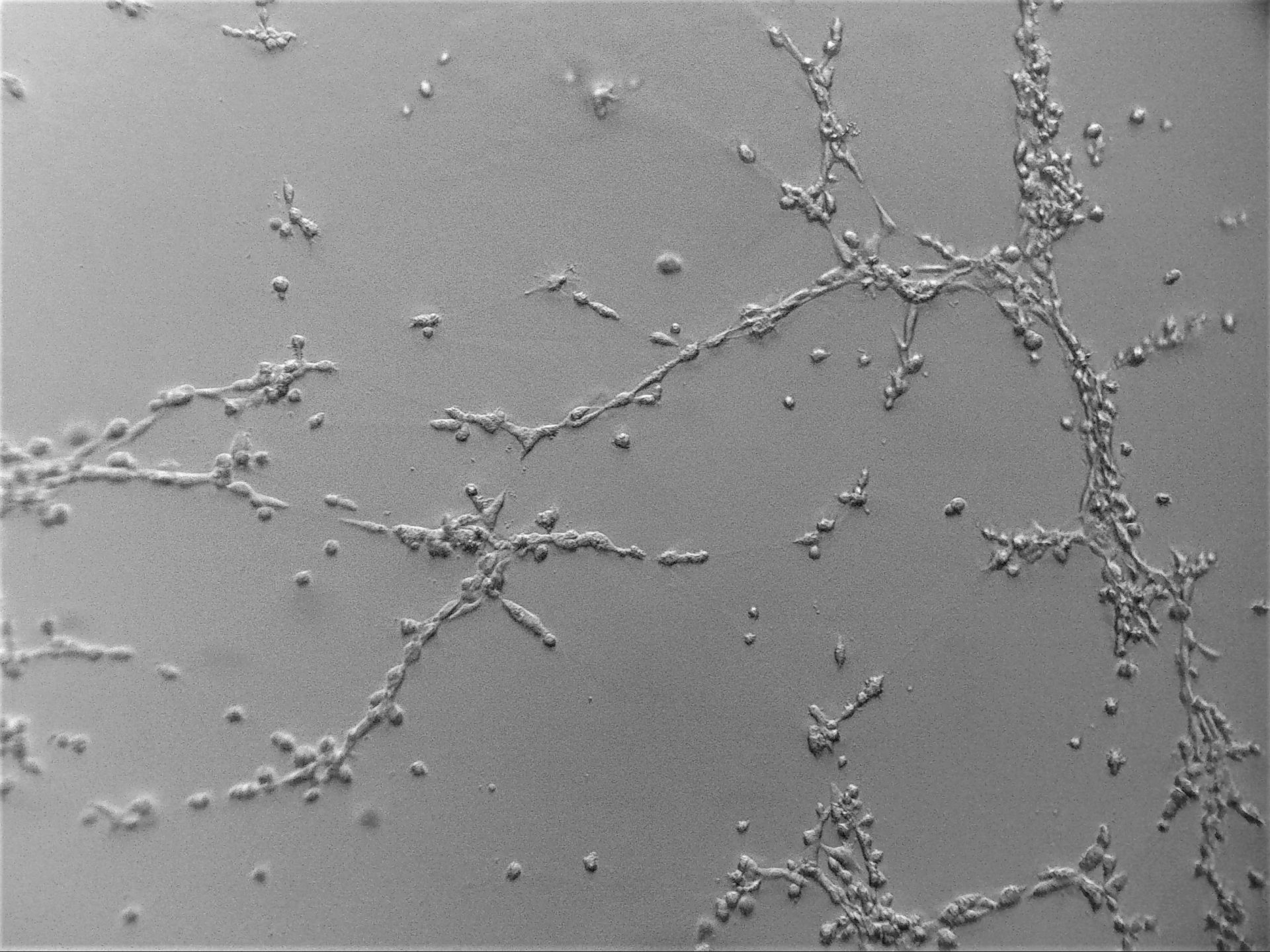
U-2 OS
bone carcinoma
Photos below show U-2 OS cells with stable transfection of GFP growing on LifeGel. Cells have been cultured on 96‑well plate for 21 days, 500 cells per well was seeded (obj. 2,5x lens 1,6x).
HUVEC
Human umbilical vein endothelial cells for angiogenesis research
HEK 293
human embryonic kidney 293 cells
HEK 293 cells grown on LifeGel was triple stained with DiO for cell membranes (green colour), MitoLite for mitochondria (red colour) and Hoechst for nuclei (blue colour). Photos: obj. 10x lens 2,5x.